Mineral elements.doc
Minerals1. The role of mineral elements in the human body 1
2. Macronutrients, their characteristics
3. Trace elements, their characteristics
4. Influence of technological processing
On the mineral composition of food products
5. Methods for the determination of mineral substances
1. The role of mineral elements in the human body
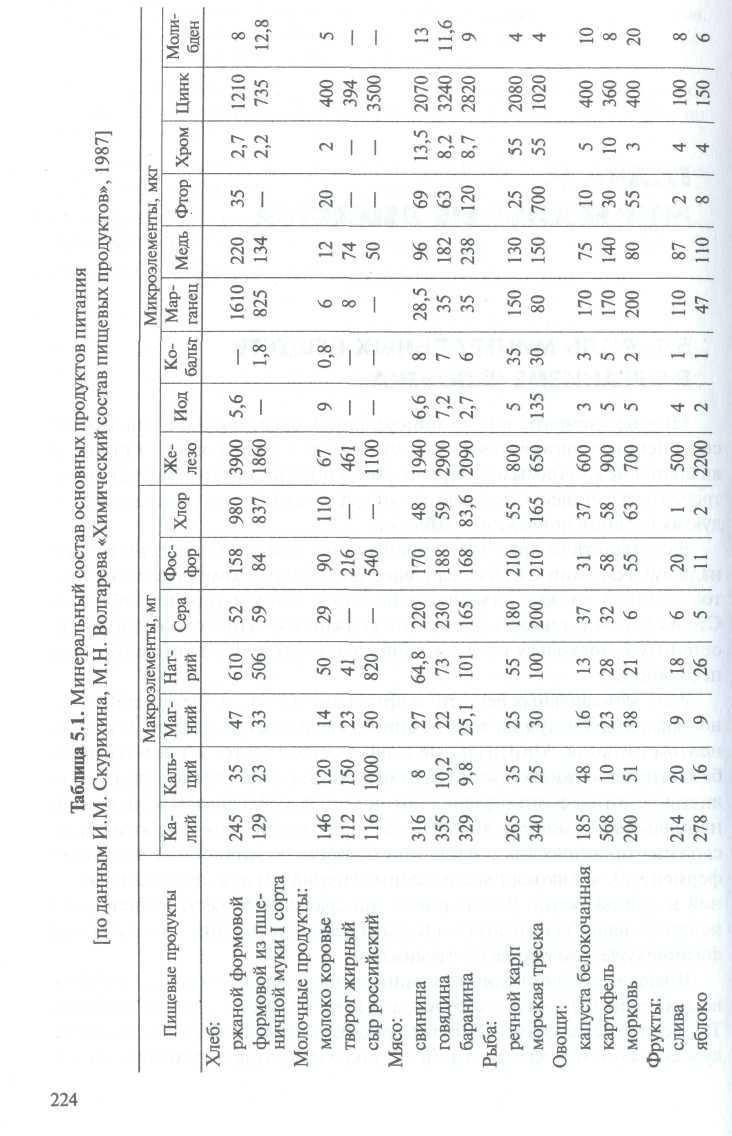
Many elements in the form of mineral salts, ions, complex compounds and organic matter are part of living matter and are essential nutrients that must be consumed daily with food. The content of minerals in the main foodstuffs is given in table. 5.1.
How to determine the lack of magnesium in the body?
The first sign of essential metals is the reversal of symptoms and the restoration of optimal growth in cattle. Over time, biochemical studies led to the isolation of enzymes that required the metal ions to function, and soon after, these specific enzymes could be associated with deficiency symptoms.
Changes in the digestive system
Metal ion interactions were considered as detrimental as well as valuable for the system. For example, an early study showed that copper enhanced the effects of iron in alleviating anemia in laboratory rats fed a milk-based diet; this observation was repeated in chickens and pigs and soon attracted the attention of clinicians who adopted a similar bimetallic protocol for the treatment of anemic humans. Along with the advent of semi-purified diets at the same time, nutritional science was on the threshold of important discoveries about the role of essential mineral elements.
As recommended by the Dietetic Board National Academy USA, the daily intake of chemical elements with food should be at a certain level (Table 5.2). The same number of chemical elements must be excreted daily from the body, since their content in it is relatively constant.
Mineral cofactors contain large group inorganic substances with most metal ions. The metal ion domain includes macrometals, trace metal ions, and metalloids. In looking for the reason for their necessity, we must understand that metal ions are suitable for performing dangerous chemical reactions on enzymatic surfaces, reactions that could damage the more sensitive organic amino acid side chains in the enzyme. For example, redox metals such as iron, manganese, and copper can accept electrons in their structure, temporarily holding them, and then transfer them to oxygen to form water as a way to safely remove an electron.
The role of minerals in the human body is extremely diverse, despite the fact that they are not an essential component of nutrition. Mineral substances are contained in protoplasm and biological fluids, they play a major role in ensuring the constancy of osmotic pressure, which is a necessary condition for the normal functioning of cells and tissues. They are part of complex organic compounds(for example, hemoglobin, hormones, enzymes), are a plastic material for building bone and dental tissue. In the form of ions, mineral substances are involved in the transmission of nerve impulses, provide blood clotting and other physiological processes of the body.
In essence, it should be taken into account that the metal cofactor expands the repertoire of available catalytic functions and is performed by enzymes. Enzymes that depend on metal ions as cofactors fall into 2 categories: metal activated enzymes and metalloenzymes. As the name suggests, metal-activated enzymes are spurred to higher catalytic activity by the presence of a mono or divalent metal ion on the outside of the protein. The metal can activate the substrate, bind the enzyme directly, or come into equilibrium with the enzyme using its ionic charge to get a more favorable bond with the substrate or a better catalytic environment.
 |
Depending on the amount of minerals in the human body and food products, they are divided into macro- and trace elements. So, if the mass fraction of an element in the body exceeds 10 -2%, then it should be considered a macroelement. The proportion of trace elements in the body is 10 -3 -10 -5%. If the content of an element is below 10 -5%, it is considered an ultramicroelement. Macronutrients include potassium, sodium, calcium, magnesium, phosphorus, chlorine and sulfur. They are contained in amounts measured in hundreds and tens of milligrams per 100 g of tissue or food. Trace elements are part of the tissues of the body in concentrations expressed in tenths, hundredths and thousandths of a milligram and are necessary for its normal functioning. Trace elements are conditionally divided into two groups: absolutely or vital (cobalt, iron, copper, zinc, manganese, iodine, bromine, fluorine) and so-called probably necessary (aluminum, strontium, molybdenum, selenium, nickel, vanadium and some others). Trace elements are called vital if, in their absence or deficiency, the normal functioning of the body is disrupted.
The distribution of trace elements in the body depends on their chemical properties and is very diverse. Iron, for example, is an integral part of hemoglobin, myoglobin and other respiratory pigments, that is, substances involved in the absorption and transport of oxygen to all tissues of the body; copper atoms are included in the active center of a number of enzymes, etc.
Therefore, metal-activated enzymes require the metal to be present in excess, perhaps 2-10 times the concentration of the enzyme. Because the metal cannot bind in a more permanent manner, metal-activated enzymes typically lose activity during purification.
In contrast, metal enzymes have a metal cofactor tightly bound to a specific region on the surface of the protein. With few exceptions, trace metals enter the picture as cofactors for metal enzymes. A strong union makes it impossible for the metal ion to be lost by dialysis or lost by weak dissociative agents. Metal enzymes, however, can lose their metal cofactor and become inactive when treated with metal chelators, which have a stronger binding affinity than the enzyme and overcome the enzyme protein by the metal ion.
The action of microelements can also be indirect - through the influence on the intensity or nature of metabolism. So, some microelements (for example, manganese, zinc, iodine) affect growth, and their insufficient intake with food inhibits normal physical development child. Other trace elements (for example, molybdenum, copper, manganese) are involved in the reproductive function, and their deficiency in the body negatively affects this side of human life.
As prosthetic groups, metals in metalloenzymes have a stoichiometric ratio represented by a full integrator. Metalloenzymes are rarely prepared to increase activity by adding their conjugated metal ion to the enzyme. Spatial geometry is also a concern: the metals in the first series of transients must adhere to strict geometrical configurations around the metal bonding site.
With the exception of those with zinc, enzymes with metals from the first transient series tend to be very bright; for example, the red color of hemoglobin or Blue colour ceruloplasmin associated with copper. Most enzymes combine iron with iron either as heme or as a special arrangement of iron with sulfur groups, known as iron-sulfur centers. Iron in heme shows a strong affinity for magnesium ions in chlorophyll. Heme, which is basically a porphyrin ring system with iron located in the center, is the most abundant form of iron in biological proteins.
To the most deficient minerals in the diet modern man include calcium and iron, excess - sodium and phosphorus.
Lack or excess in the diet of any mineral substances causes a violation of the metabolism of proteins, fats, carbohydrates, vitamins, which leads to the development of a number of diseases. Below are the characteristic (typical) symptoms of a deficiency of various chemical elements in the human body: The most common consequence of a discrepancy in the amount of calcium and phosphorus in the diet is dental caries, bone thinning. With a lack of fluoride in drinking water, tooth enamel is destroyed, iodine deficiency in food and water leads to thyroid diseases. Thus, minerals are very important for the elimination and prevention of a number of diseases.
Most Common Connections
As a component of the iron-sulfur centers, iron enters several group schemes with cysteine residues in enzymes that allow for more direct contact with the protein. The iron at these centers binds to substrates as well as transfers electrons and participates in reactions that include dehydration and rearrangement. Enzymes with iron-sulfur centers include xanthine oxidase, succinate dehydrogenase, aconitase, and nitric acid.
This arrangement allows the enzyme to remove a hydrogen atom from a very stable S-N connections. A non-metal can replace iron in these complexes. Enzymes with a heme group are usually reddish brown in color. The color motivated initial interest in these proteins and was a motivating factor for labeling heme proteins in mitochondria as "cytochromes".


We list the causes of metabolic disorders of mineral substances that can occur even with their sufficient amount in food:
A) unbalanced nutrition (insufficient or excessive amounts of proteins, fats, carbohydrates, vitamins, etc.);
Although only a few soluble enzymes have iron as a cofactor, iron is especially prominent in membrane-bound proteins that contain electron transport pathways. The redox property of iron plays a large part of its chemistry as a cofactor. Iron is almost always involved in electron transfer and often donates electrons to an oxygen molecule.
Both catalase and peroxidase, two heme enzymes, use iron to interact with dangerous oxidants. Both enzymes are located in the cytosol and in peroxisomes, where harmful oxidation reactions occur during normal metabolic events. Perhaps the most familiar iron-containing enzyme is cytochrome c oxidase, the terminal electron acceptor in the mitochondrial electron transport chain and an enzyme capable of splitting an oxygen molecule to form water.
B) the use of methods of culinary processing of food, causing the loss of minerals, for example, during defrosting (in hot water) meat, fish, or when removing decoctions of vegetables and fruits, where soluble salts pass;
C) the lack of timely correction of the composition of diets when the body's need for minerals associated with physiological reasons changes. For example, people working in conditions elevated temperature the external environment, the need for potassium, sodium, chlorine and other minerals increases due to the fact that most of them are excreted from the body with sweat;
The role of magnesium in the human body
Zinc is perhaps the most abundant and versatile of all metal cofactors. More than 300 enzymes have a zinc cofactor. Approximately 3% of the mammalian genome encodes zinc finger proteins. As a cofactor, zinc can perform both structural and catalytic functions. These examples illustrate why zinc is an important companion for enzymes and proteins.
Zinc is considered a soft metal because it behaves like a divalent cation without much geometric preference. Perhaps this softness allows zinc to adapt to many different fermentation environments. For this reason, zinc complexes are colorless and zinc itself behaves primarily as a cation. Another example is the use of zinc to polarize an ester or amide bond, thereby facilitating the nucleophilic attack of water on the compound, as in carboxypeptidase and aminopeptidase catalyzed reactions.
D) violation of the process of absorption of minerals in the gastrointestinal tract or increased fluid loss (for example, blood loss).
^
2. Macronutrients, their characteristics
Calcium. It is the main structural component of bones and teeth; is part of the nuclei of cells, cell and tissue fluids, is necessary for blood clotting. Calcium forms compounds with proteins, phospholipids, organic acids; participates in the regulation of the permeability of cell membranes, in the transmission of nerve impulses, in the molecular mechanism of muscle contractions, controls the activity of a number of enzymes. So calcium performs not only plastic functions, but also affects many biochemical and physiological processes in the body.
Copper, like iron, is a redox metal. Copper enzymes, although not as numerous as zinc enzymes, perform important biological functions, mainly in the cytosol. The most complex enzymes include multicorex oxidases, which can have as few as 4 or as many as 8 copper atoms per enzyme. The copper in these enzymes exists in three different chemical environments, known as type 1, type 2, and type copper patches. The copper type 1 site gives blue color to ceruloplasmins and other blue proteins with copper.
The copper binding sites in the polyoxide oxidase form a triad consisting of type 3 copper 2 and type 3 copper arranged in an isosceles triangle. Oxygen binds to these two type 3 medics at the base of the triangle. Because of its tendency to accept electrons, copper is a powerful oxidizing agent in biological systems. This reaction links iron metabolism to copper and may explain how a lack of copper in iron prevents iron transfer and causes anemia in humans. Rarely copper is called upon to play only a structural role, and many enzymes that have copper as a cofactor use the metal at the active site.
Calcium is a hard-to-digest element. Calcium compounds entering the human body with food are practically insoluble in water. The alkaline environment of the small intestine promotes the formation of indigestible calcium compounds, and only the action of bile acids ensures its absorption.
The assimilation of calcium by tissues depends not only on its content in foods, but also on its ratio with other food components and, first of all, with fats, magnesium, phosphorus, and proteins. With an excess of fat, there is competition for bile acids and a significant part of the calcium is excreted from the body through the large intestine. Absorption of calcium is adversely affected by an excess of magnesium; the recommended ratio of these elements is 1:0.5. If the amount of phosphorus exceeds the level of calcium in food by more than 2 times, then soluble salts are formed, which are extracted by the blood from the bone tissue. Calcium enters the walls of blood vessels, which causes their fragility, as well as into the tissues of the kidneys, which can contribute to the occurrence of kidney stones. For adults, the recommended ratio of calcium and phosphorus in food is 1:1.5. The difficulty in maintaining this ratio is due to the fact that most commonly consumed foods are much richer in phosphorus than in calcium. Phytin and oxalic acid, contained in a number of plant products, have a negative effect on the absorption of calcium. These compounds form insoluble salts with calcium.
Research has linked copper ions to artery formation or angiogenesis. One of the most exciting discoveries, which has yet to be fully understood, is that depriving an animal of copper delays or even inhibits the growth of cancerous tumors. Nutritionally, this may mean that copper is essential for microvascular development.
Do you know that
While zinc may be the most common transition metal in enzymes, manganese is perhaps the least common, in part because manganese complexes with proteins tend to be weakly stable and readily dissociate. Known manganese metalloenzymes include pyruvate carboxylase and manganese superoxide dismutase in mitochondria and arginase in the urea cycle. Manganese may also function as a metal-activating cofactor for many enzymes that require magnesium.
daily requirement in adult calcium is 800 mg, and in children and adolescents - 1000 mg or more.
With insufficient intake of calcium or in violation of its absorption in the body (with a lack of vitamin D), a state of calcium deficiency develops. There is an increased excretion of it from bones and teeth. In adults, osteoporosis develops - demineralization of bone tissue, in children, the formation of the skeleton is disturbed, rickets develops.
Features of nutrition with a lack of magnesium
Although manganese is not considered a redox metal based on its reactivity, it can nevertheless exist in 6 oxidation states, three of which are not observed in biological systems. The cobalt is attached in a square, flat arrangement to a ring similar to that of the gay, but with very special characteristics. Unlike heme, cobalt has 2 axial ligands that are free of protein, allowing protein groups to access the central metal above and below the plane.
The best sources of calcium are milk and dairy products, various cheeses and cottage cheese (100-1000 mg / 100 g of product), green onions, parsley, beans. Significantly less calcium is found in eggs, meat, fish, vegetables, fruits, berries (20-40 mg / 100 g of product).
Magnesium. This element is required for the activity of a number of key enzymes. for the body's metabolism. Magnesium is involved in maintaining the normal function of the nervous system and heart muscles; has a vasodilating effect; stimulates bile secretion; raises motor activity intestines, which helps to remove toxins from the body (including cholesterol).
Why is magnesium deficiency dangerous for pregnant women?
In one octahedral complex, one axial position is usually occupied by one benzimidazole and the other by a methyl group. The device is unique and allows cobalt to form carbon-metal bonds with the potential for two different reactions. For example, a methyl group can be removed as a carbonium ion by holding both electrons in cobalt, which then reverts to a less stable one.
In positional permutations, cobalt retains only one electron and forms a stable coion 7 with the release of a free radical. Free radicals are highly reactive and overcome energy barriers that other reactants can hold. In this way, Chemical properties cobalt transfer groups such as carbonium ions or highly reactive carbon-centered radicals. Both products are possible and explain the need for cobalt as a cofactor for the reaction to proceed via the free radical mechanism.
Magnesium absorption is hindered by the presence of phytin and excess fat and calcium in food. The daily requirement for magnesium is not precisely defined; it is believed, however, that a dose of 200-300 mg / day prevents the manifestation of deficiency (it is assumed that about 30% of magnesium is absorbed).
With a lack of magnesium, the absorption of food is disrupted, growth is delayed, calcium is deposited in the walls of blood vessels, and a number of other pathological phenomena develop. In humans, a lack of magnesium ions, due to the nature of nutrition, is extremely unlikely. However, large losses of this element can occur with diarrhea; their consequences are felt if fluids that do not contain magnesium are introduced into the body. When the serum magnesium concentration drops to about 0.1 mmol / l, a syndrome resembling delirium tremens may occur: a person has a semi-comatose state, muscle tremors, muscle spasms in the wrist and foot, increased neuromuscular excitability in response to sound , mechanical and visual stimuli. The introduction of magnesium causes a rapid improvement in the condition.
Magnesium is rich mainly in plant foods. A large amount of it contains wheat bran, various cereals (40 - 200 mg / 100 g of product), legumes, apricots, dried apricots, prunes. There is little magnesium in dairy products, meat, fish, pasta, most vegetables and fruits (20 - 40 mg / 100 g).
Potassium. About 90% of potassium is inside the cells. It, together with other salts, provides osmotic pressure; participates in the transmission of nerve impulses; regulation of water-salt metabolism; promotes the removal of water, and, consequently, toxins from the body; maintains the acid-base balance of the internal environment of the body; participates in the regulation of the activity of the heart and other organs; necessary for the functioning of a number of enzymes.
Potassium is well absorbed from the intestines, and its excess is quickly removed from the body with urine. The daily requirement for potassium in an adult is 2000-4000 mg. It increases with profuse sweating, with the use of diuretics, diseases of the heart and liver. Potassium is not a deficient nutrient in the diet, and with a varied diet, potassium deficiency does not occur. Potassium deficiency in the body appears when the function of the neuromuscular and cardiovascular systems is impaired, drowsiness, decreased blood pressure, cardiac arrhythmias. In such cases, a potassium diet is prescribed.
Most potassium comes from plant foods. Its rich sources are apricots, prunes, raisins, spinach, seaweed, beans, peas, potatoes, other vegetables and fruits (100 - 600 mg / 100 g of product). Less potassium is found in sour cream, rice, bread made from premium flour (100 - 200 mg / 100 g).
Sodium. Sodium is found in all tissues and body fluids. He is involved in maintaining osmotic pressure in tissue fluids and blood; in the transmission of nerve impulses; regulation of acid-base balance, water-salt metabolism; increases the activity of digestive enzymes.
Sodium metabolism has been extensively studied due to its physiological properties and importance to the body. This nutrient is easily absorbed from the intestines. Sodium ions cause swelling of tissue colloids, which causes water retention in the body and counteracts its release. The total amount of sodium in the extracellular fluid thus determines the volume of these fluids. An increase in plasma sodium concentration leads to a feeling of thirst. In a hot climate and during hard physical work, there is a significant loss of sodium with sweat and it is necessary to introduce salt into the body to make up for the lost amount.
Basically, sodium ions enter the body at the expense of table salt - NaCl. With excessive consumption of sodium chloride, the removal of water-soluble end products of metabolism through the kidneys, skin and other excretory organs worsens. Water retention in the body complicates the activity of the cardiovascular system, increases blood pressure. Therefore, the consumption of salt in the relevant diseases in the diet is limited. However, when working in hot shops or hot climates, the amount of sodium (in the form of table salt) introduced from the outside is increased to compensate for its loss with sweat and reduce sweating, which burdens the function of the heart.
Sodium is naturally present in all foods. The method of obtaining food products largely determines the final content of sodium in it. For example, frozen green peas contain much more sodium than fresh ones. Fresh vegetables and fruits contain less than 10 mg/kg to 1 g/kg, unlike cereals and cheese, which can contain sodium in amounts of 10-20 g/kg.
Estimating the average daily intake of sodium from food is difficult because sodium concentration in food varies widely and, in addition, people are used to adding salt to food. An adult consumes up to 15 g of table salt daily and excretes the same amount from the body. This amount is much higher than the physiologically necessary and is determined primarily by the taste of sodium chloride, the habit of salty foods. The content of table salt in human food can be reduced to 5 g per day without harm to health. The release of sodium chloride from the body, and, consequently, the need for it, is affected by the amount of potassium salts received by the body. Plant foods, especially potatoes, are rich in potassium and increase the excretion of sodium chloride in the urine, and, consequently, increase the need for it.
Phosphorus. Phosphorus is found in all tissues of the body, especially muscles and the brain. This element is involved in all life processes of the body. : synthesis and breakdown of substances in cells; regulation of metabolism; is a part of nucleic acids and a number of enzymes; needed for the formation of ATP.
Phosphorus is found in body tissues and food products in the form of phosphoric acid and its organic compounds (phosphates). Its main mass is in the bone tissue in the form of calcium phosphate, the rest of the phosphorus is part of the soft tissues and fluids. In the muscles, the most intensive exchange of phosphorus compounds occurs. Phosphoric acid is involved in the construction of the molecules of many enzymes, nucleic acids, etc.
With a long-term deficiency of phosphorus in the diet, the body uses its own phosphorus from bone tissue. This leads to demineralization of bones and a violation of their structure - rarefaction. When the body is depleted of phosphorus, mental and physical performance decreases, loss of appetite, apathy is noted.
The daily requirement for phosphorus for adults is 1200 mg. It increases with great physical or mental stress, with certain diseases.
A large amount of phosphorus is found in animal products, especially in the liver, caviar, as well as in cereals and legumes. Its content in these products ranges from 100 to 500 mg per 100 g of product. Cereals (oatmeal, pearl barley) are a rich source of phosphorus, they contain 300-350 mg of phosphorus / 100 g. However, phosphorus compounds are absorbed from plant products worse than when eating food of animal origin.
Sulfur. The importance of this element in nutrition is determined, first of all, by the fact that it is part of proteins in the form of sulfur-containing amino acids. (methionine and cystine), and is also an integral part of some hormones and vitamins.
As a component of sulfur-containing amino acids, sulfur is involved in the processes of protein metabolism, and the need for it increases sharply during pregnancy and body growth, accompanied by the active inclusion of proteins in the resulting tissues, as well as during inflammatory processes. Sulfur-containing amino acids, especially in combination with vitamins C and E, have a pronounced antioxidant effect. Along with zinc and silicon, sulfur determines the functional state of hair and skin.
Chlorine. This element is involved in the formation of gastric juice, the formation of plasma, activates a number of enzymes. This nutrient is easily absorbed from the intestines into the blood. The ability of chlorine to be deposited in the skin, to linger in the body with excessive intake, and to be excreted with sweat in significant quantities is interesting. The excretion of chlorine from the body occurs mainly with urine (90%) and sweat.
Violations in the exchange of chlorine lead to the development of edema, insufficient secretion of gastric juice, etc. A sharp decrease in the content of chlorine in the body can lead to a serious condition, even death. An increase in its concentration in the blood occurs with dehydration of the body, as well as with a violation of the excretory function of the kidneys.
The daily requirement for chlorine is approximately 5000 mg. Chlorine enters the human body mainly in the form of sodium chloride when added to food.
^
3. Trace elements, their characteristics
Iron. This element is necessary for the biosynthesis of compounds that provide respiration, hematopoiesis; it participates in immunobiological and redox reactions; is part of the cytoplasm, cell nuclei and a number of enzymes.
Iron assimilation is prevented by oxalic acid and phytin. For the assimilation of this nutrient, vitamin B 12 is required. Ascorbic acid also contributes to the absorption of iron, since iron is absorbed in the form of a divalent ion.
^ A lack of iron in the body can lead to the development of anemia, gas exchange, cellular respiration, that is, the fundamental processes that ensure life, are disrupted. The development of iron deficiency states is facilitated by: insufficient intake of iron in the body in an assimilated form, a decrease in the secretory activity of the stomach, a deficiency of vitamins (especially B 12 , folic and ascorbic acids) and a number of diseases that cause blood loss.
The iron requirement of an adult (14 mg/day) is met in excess by the normal diet. However, when bread from fine flour containing little iron is used in food, iron deficiency is very often observed in urban residents. At the same time, it should be taken into account that grain products rich in phosphates and phytin form sparingly soluble compounds with iron and reduce its assimilation by the body.
Iron is a widespread element. It is found in offal, meat, eggs, beans, vegetables, berries. However, in an easily digestible form, iron is found only in meat products, liver (up to 2000 mg / 100 g of product), egg yolk.
Copper. Copper is an essential element in human metabolism, playing a role in the formation of red blood cells, the release of tissue iron, and the development of the skeleton, central nervous system, and connective tissue.
Since copper is widely distributed in foods, it is unlikely that people, with the possible exception of infants, on a purely dairy diet, will ever develop a form of copper-related malnutrition.
The consumption of excessively large doses of copper by a person leads to irritation and erosion of the mucous membranes, widespread damage to the capillaries, damage to the liver and kidneys, and irritation of the central nervous system. The daily requirement for this element is about 2 mg. Sources of copper are foods such as liver, egg yolk, green vegetables.
Iodine. Iodine is an essential element involved in the formation of the hormone thyroxine. With iodine deficiency, goiter develops - a disease of the thyroid gland.
The need for iodine ranges from 100-150 mcg per day. The content of iodine in foodstuffs is usually low (4-15 µg%). Sea foods are the richest in iodine. So, in sea fish it contains about 50 mcg / 100 g, in cod liver up to 800, in seaweed, depending on the type and timing of collection - from 50 mcg to 70,000 mcg / 100 g of the product. But it must be taken into account that during long-term storage and heat treatment of food, a significant part of iodine (from 20 to 60%) is lost.
The content of iodine in terrestrial plant and animal products is highly dependent on its amount in the soil. In areas where there is little iodine in the soil, its content in food products can be 10 to 100 times less than the average. Therefore, in these areas to prevent goiter, a small amount of potassium iodate (25 mg per 1 kg of salt) is added to table salt. The shelf life of such iodized salt is no more than 6 months, since iodine gradually disappears during salt storage.
Fluorine. With a lack of this element, dental caries develops (destruction of tooth enamel). An excess of fluorine also has a negative effect on the body, since fluorine salts, accumulating in the bones, cause a change in the color and shape of the teeth, osteochondrosis, and after this coarsening of the joints and their immobility, bone growths. The difference between useful and harmful doses of fluorine is so small that many researchers oppose water fluoridation.
Fluorine consumed with water is almost completely absorbed; fluorine contained in food is absorbed to a lesser extent. Absorbed fluorine is evenly distributed throughout the body. It is retained mainly in the skeleton, and a small amount is deposited in the dental tissue. In high doses, fluorine can cause a violation of carbohydrate, lipid, protein metabolism, as well as the metabolism of vitamins, enzymes and mineral salts.
Estimates of the daily intake of fluoride from food have been made in various countries; for adults, this value varies from 0.2 to 3.1 mg, for children age group from 1 to 3 years, the intake of fluoride was estimated at 0.5 mg/day.
Almost all food products contain at least trace amounts of this element. All types of vegetation contain some amount of fluorine, which they obtain from the soil and water. High levels of fluoride have been found in certain foods, particularly fish, some vegetables and tea. The use of fluoridated water in food processing plants can often double the level of fluoride in finished products.
For the prevention and treatment of dental caries, various toothpastes, powders, elixirs, chewing gums and the like, which contain added fluorine, mainly in inorganic form. These compounds are commonly incorporated into dentifrices, typically at concentrations of about 1 g/kg.
Chromium. This element seems to be essential for glucose and lipid metabolism and for the utilization of amino acids by some systems. He also has importance for the prevention of mild forms of diabetes and atherosclerosis in humans.
Chromium is absorbed both from the gastrointestinal tract and from the respiratory tract. The absorbed amount is not the same for each of these systems and depends on the form of chromium. Trivalent chromium is the essential form of the element for humans, hexavalent chromium is toxic. Chromium is distributed throughout the tissues of the human body in unequal, but usually low concentrations. Chromium levels in all tissues except the lungs decrease with age. The greatest amounts of chromium in humans accumulate in the skin, muscles and adipose tissue. Homeostatic mechanisms, including mechanisms of transport in the liver and intestines, prevent the excessive accumulation of trivalent chromium. Chromium is slowly excreted from the body, mainly in the urine.
Today it is considered to be the norm of consumption of about 150 mg of chromium per day. It is especially useful for older people whose body does not absorb carbohydrates well, and chromium enhances the metabolic processes of these particular compounds. Inorganic chromium is absorbed poorly, much easier - in organic compounds, that is, in the form in which it is found in living organisms.
Food products vary considerably in chromium levels, which range from 20 to 550 µg/kg. Rich sources of chromium are brewer's yeast, liver (10-80 mcg/100 g). In smaller quantities, this element is found in potatoes with skins, beef, fresh vegetables, wholemeal bread, cheese.
Manganese. Manganese is essential as a cofactor in a number of enzyme systems; it plays a role in the proper functioning of flavoproteins, in the synthesis of sulfated mucopolysaccharides, cholesterol, hemoglobin and in many other metabolic processes. Of the ingested manganese, only about 3% is absorbed.
The absorption of manganese is closely related to the absorption of iron. The need for manganese is 0.2-0.3 mg per 1 kg of human weight per day. Most manganese is found in cranberries and tea, a little less in chestnuts, cocoa, vegetables, fruits (100-200 mcg / 100 g).
^ Nickel. Nickel has been recognized as an essential trace element relatively recently. At present, its role as a coenzyme in the processes of iron metabolism has been established. At the same time, an increase in the intake of iron in the body is accompanied by an increase in the need for food nickel. In addition, nickel contributes to the absorption of copper - another element indispensable for hematopoiesis. The importance of food nickel or nickel isolated from natural products is emphasized by the fact that synthetic compounds of this element are carcinogenic.
Nickel is present in most foods, but at concentrations below (and often much below) 1 mg/kg. Dietary intake of nickel has been reported to range from less than 200 to 900 µg/day. With a normal diet, about 400 mcg / day comes in. It has been shown that the content of nickel in wines and beer is 100 and 50 µg/L, respectively.
Zinc. This trace element as a coenzyme is involved in a wide range of reactions of protein biosynthesis (more than 70) and nucleic acid metabolism (including the processes of DNA replication and transcription), which primarily ensure the growth and puberty of the body. At the same time, zinc, along with manganese, is a specific trace element that affects the state of sexual function, namely, the activity of some sex hormones, spermatogenesis, the development of male gonads and secondary sexual characteristics. In addition, the role of zinc in the prevention of hypertrophic processes in the prostate gland has recently been considered.
Zinc, together with sulfur, is involved in the growth and renewal of skin and hair. Along with manganese and copper, zinc contributes significantly to the perception of taste and smell sensations. Zinc as an indispensable component is part of the insulin molecule, and its level is reduced in patients with diabetes mellitus. It is very important that this trace element is a coenzyme of alcohol dehydrogenase, which ensures the metabolism of ethyl alcohol. At the same time, the level of absorption of zinc in chronic alcoholism is sharply reduced. The so-called "night blindness" (i.e., impaired night vision) can develop not only in the absence of vitamin A, but also zinc. Zinc, together with vitamin B 6, ensures the metabolism of unsaturated fatty acids and the synthesis of prostaglandins.
Zinc is very important for digestion and nutrient absorption. So, zinc provides the synthesis of the most important digestive enzymes in the pancreas, and also participates in the formation of chylomicrons - transport particles, in which dietary fats can be absorbed into the blood. Zinc, along with B vitamins, is an important regulator of the functions of the nervous system. Under conditions of zinc deficiency, emotional disorders, emotional instability, irritability, and in very severe cases, cerebellar dysfunction can occur. Finally, more and more data are accumulating in favor of the participation of zinc in the processes of maturation of lymphocytes and reactions of cellular immunity.
The daily requirement for zinc is 8000-22000 mcg%. She is quite satisfied with the usual diet. The average daily intake of zinc with drinking water alone is about 400 mcg. The content of zinc in food products usually ranges from 150-25000 mcg%. However, in the liver, meat and legumes, it reaches 3000 - 5000 mcg%. Sometimes, zinc deficiency can be experienced by the body of children and adolescents who do not consume enough animal products.
^ Selenium. Even in the middle of the XX century. selenium was not only not considered by nutritional science, but was even considered a very toxic element with carcinogenic properties. However, already in the 60s. it was found that with a lack of selenium, the cardiovascular system suffers, which is manifested by progressive atherosclerosis and weakness of the heart muscle, and in conditions of chronic selenium deficiency, almost incurable cardiomyopathy can develop. Lately at the level contemporary research finds confirmation of one of the important observations of ancient Chinese medicine, indicating that adequate provision of the body with selenium helps to slow down the aging process and leads to longevity . It is interesting to note that the famous medicinal varieties of green tea, supplied with the aim of achieving health and longevity in the imperial palaces in Ancient China, were grown in those mountainous provinces, in the soils of which high selenium content is already determined using modern analytical methods.
After the discovery of selenium, it was found that vitamin E and selenium act on different parts of the same process and are strictly complementary to each other, that is, their antioxidant activity increases dramatically when used together. The synergy of both antioxidants is of particular interest in the context of anticancer activity. Thus, it was shown that the administration of selenium preparations simultaneously with vitamin E significantly increased the anticarcinogenic effect in relation to experimental tumors.
The intake of selenium with food depends on the conditions and nature of food intake and the level of selenium in food products. Vegetables and fruits are generally a poor source of selenium, in contrast to grains, grain products, meat (especially by-products), seafood, which contain significant amounts of selenium, typically well over 0.2 mg/kg wet weight . Chemical composition Soil and the content of selenium in it significantly affect the amount of selenium in the grain, varying from 0.04 mg/kg to 21 mg/kg.
Molybdenum. The total amount of molybdenum in the body of an adult is about 7 mg. The content of molybdenum in the blood is about 0.5 micrograms per 100 ml. Higher concentrations of this element have been found in people living in regions where the soil is most rich in compounds of this metal. Thus, in some regions of Armenia, frequent cases of gout have been noted among residents who eat mainly local products, in which extremely high levels of molybdenum have been found. Its content in the diet of the inhabitants of this region was 10-15 mg. In other areas where cases of gout were less common, people received only 1-2 mg of molybdenum per day from food.
Molybdenum is an integral part of a number of enzymes, such as xanthine oxidase, aldehyde oxidase, sulfate oxidase. It is known that molybdenum inhibits the development of caries.
The estimated daily requirement for molybdenum is 2 mcg per 1 kg of body weight. In Russia, the daily intake of molybdenum is 0.27 mg.
richest in molybdenum different kinds vegetables (such as legumes) and the internal organs of animals.
Cobalt. The biological effect of cobalt has been known since 1948, when scientists Rickes and Smith found that the cobalt atom is central in the vitamin B 12 molecule. The maximum concentration of cobalt in tissues is about 100 μg / kg. The total content of cobalt in the body of an adult is 5 mg. A person with food daily receives 5.63 -7.94 micrograms of cobalt, of which 73 - 97% is absorbed.
The average daily requirement for cobalt is 60 mcg per 1 kg of body weight. It is believed that a person needs cobalt only in the form of cyanocobalamin (vitamin B 12). In some countries, cobalt compounds have been used as a food additive to beer to stabilize the foam. However, it turned out that such an additive was the cause of heart disease in beer consumers. Therefore, the use of cobalt compounds as a food additive has now been abandoned.
^
4 Effect of processing on the mineral composition of foods
When processing food raw materials, as a rule, there is a decrease in the content of mineral substances (except for Na, added in the form of food salt). In plant foods, they are lost with waste. Thus, the content of a number of macro- and especially microelements during the production of cereals and flour after grain processing decreases, since there are more of these components in the removed shells and germs than in the whole grain. Comparative analysis mineral composition in wheat flour of the highest grade and flour from whole grains is given below (the content of elements is indicated in mg / 100 g of product):
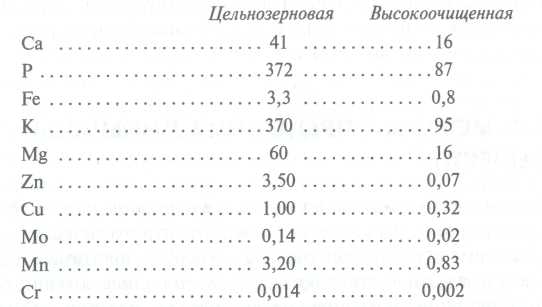
For example, on average, the grain of wheat and rye contains about 1.7% of ash elements, while in flour, depending on the variety, from 0.5 (in the highest grade) to 1.5% (in the wholemeal). When cleaning vegetables and potatoes, 10 to 30% of minerals are lost. If they are subjected to heat cooking, then depending on the technology (cooking, frying, stewing), another 5 to 30% is lost.
Meat, fish products and poultry mostly lose macronutrients such as calcium and phosphorus during the separation of the pulp from the bones.
During thermal cooking (boiling, frying, stewing), meat loses from 5 to 50% of minerals. However, if the processing is carried out in the presence of bones containing a lot of calcium, it is possible to increase the calcium content in cooked meat products by 20%.
In the technological process, due to insufficiently high-quality equipment, a certain amount of microelements can pass into the final product. So, when making bread during dough preparation as a result of contact of the dough with the equipment the iron content can be increased by 30%. This process is undesirable, since toxic elements contained in the form of impurities in the metal can also pass into the product along with iron. When storing canned food in prefabricated tins (that is, soldered) cans with poor-quality solder or if the protective lacquer layer is broken, highly toxic elements such as lead, cadmium, and tin can pass into the product.
It should be taken into account that a number of metals, such as iron and copper, even in small concentrations, can cause undesirable oxidation of products. Their catalytic oxidizing abilities are especially pronounced in relation to fats and fatty products. So, for example, at a concentration of iron above 1.5 mg/kg and copper 0.4 mg/kg during long-term storage of butter and margarines, these metals cause rancidity of products. When storing drinks in the presence of iron above 5 mg/l and copper 1 mg/l, under certain conditions, turbidity of drinks can often be observed.
^
5. Methods for the determination of mineral substances
For the analysis of mineral substances, physicochemical methods are mainly used - optical and electrochemical.
Almost all of these methods require special preparation of samples for analysis, which consists in the preliminary mineralization of the object of study. Mineralization can be carried out in two ways: "dry" and "wet". "Dry" mineralization involves charring, burning and calcining the test sample under certain conditions. "Wet" mineralization also provides for the processing of the object of study concentrated acids(most often HNO 3 and H 2 SO 4).
^ Spectral methods of analysis.
Photoelectrocolorimetry - analysis based on the measurement of absorption by colored solutions of monochromatic radiation in the visible region of the spectrum. Measurements are carried out using photoelectric colorimeters equipped with narrow-band filters. If the test substance is not colored, it must be converted to a colored compound by chemical reaction with certain reagents (photometric analytical reaction).
Spectrophotometry is an analysis method based on measuring the absorption of monochromatic radiation in the ultraviolet, visible and infrared regions of the spectrum. Such measurements are carried out using spectrophotometers, where dispersive prisms and diffraction gratings are used as monochromatizers.
Quantitative analysis of the ion under study is usually carried out using the calibration curve method.
Emission spectral analysis. Methods of emission spectral analysis are based on measuring the wavelength, intensity and other characteristics of light emitted by atoms and ions of a substance in a gaseous state. Emission spectral analysis makes it possible to determine the elemental composition of inorganic and organic substances.
The intensity of the spectral line is determined by the number of excited atoms in the excitation source, which depends not only on the concentration of the element in the sample, but also on the excitation conditions. With stable operation of the excitation source, the relationship between the intensity of the spectral line and the concentration of the element (if it is sufficiently small) is linear, i.e., in this case quantitative analysis can also be carried out using the calibration curve method.
The greatest application as a source of excitation received an electric arc, spark, flame. The temperature of the arc reaches 5000 - 6000°C. In an arc, it is possible to obtain the spectrum of almost all elements. With a spark discharge, a temperature of 7000 - 10000 ° C develops and all elements are excited. The flame gives a sufficiently bright and stable emission spectrum. The method of analysis using a flame as an excitation source is called flame emission analysis. This method determines more than forty elements (alkaline and alkaline earth, Cu 2 , Mn 2, etc.).
^ Atomic absorption spectroscopy . The method is based on the ability of free atoms of elements in flame gases to absorb light energy at wavelengths characteristic of each element.
In atomic absorption spectroscopy, the possibility of overlapping spectral lines of various elements is almost completely excluded, since their number in the spectrum is much less than in emission spectroscopy.
The decrease in the intensity of resonant radiation under the conditions of atomic absorption spectroscopy obeys the exponential law of decrease in intensity depending on the layer thickness and substance concentration, similar to the Bouguer-Lambert-Beer law
The constancy of the thickness of the light-absorbing layer (flame) is achieved using burners of a special design. Methods of atomic absorption spectral analysis are widely used for the analysis of almost any technical or natural object, especially in cases where it is necessary to determine small amounts of elements.
Methods for atomic absorption determination have been developed for more than 70 elements.
^ 2. Electrochemical methods of analysis.
Ionometry. The method is used to determine K ions , Na , Ca 2 , Mn 2 , F - , I - , Сl - etc.
The method is based on the use of ion-selective electrodes, the membrane of which is permeable to a certain type of ions (hence, as a rule, the high selectivity of the method).
The quantitative content of the ion being determined is carried out either using a calibration graph, which is plotted in the coordinates E - pC, or by the method of additions. The standard addition method is recommended for the determination of ions in complex systems containing high concentrations of foreign substances.
Polarography. The method of alternating current polarography is used to determine toxic elements (mercury, cadmium, lead, copper, iron).
The method is based on the study of current-voltage curves obtained during the electrolysis of an electrooxidizing or electroreducing substance. As an indicator electrode in polarography, a mercury drop electrode is most often used, sometimes solid microelectrodes - platinum, graphite. As a reference electrode, either mercury poured onto the bottom of the electrolyzer or a saturated calomel half-cell is used.
As the voltage increases, there comes a moment when all ions entering the electrode due to diffusion are immediately discharged and their concentration in the near-electrode layer becomes constant and practically equal to zero. The current flowing in the circuit at this time is called the limiting diffusion current.
Quantitative polarographic analysis is based on the use of direct proportional dependence the magnitude of the diffusion current on the concentration of the element being determined.
^ MINERAL ELEMENTS
Mineral (ash) elements are found in food products in the form of organic and inorganic compounds. They are found in many organic
substances of various classes - proteins, fats, glycosides, enzymes, etc. Usually, mineral elements are determined in the ashes after the combustion of food products, since it is quite difficult to determine exactly which substances and in what quantity these elements are included.
The role of mineral elements in the life of humans, animals and plants is enormous: all physiological processes in living organisms proceed with the participation of these elements. Thus, in the human and animal body, mineral elements are involved in plastic processes, the formation and construction of tissues, in water metabolism, in maintaining the osmotic pressure of blood and other body fluids, in maintaining acid-base balance in the body, and are included in the complex of substances that make up living protoplasm cells, in the composition of some endocrine glands, etc.
The mineral composition of organisms changes with age; with aging, mineralization of organisms is observed. So, newborn children contain about 34 g of minerals per 1 kg of body weight, in an adult, the content of these substances rises to 43 g or more.
More than 70 mineral elements have been found in the human and animal body. Many enzymatic processes occurring in various tissues of the body require the participation of a number of mineral elements. So, for the conversion of pyruvic acid into acetic acid or glucose into fructose or phosphoglycerol into glucose-6-mannose-6- and fructose-6-phosphate, the participation of magnesium ions is mandatory. Calcium ions inhibit the development of this process.
Minerals are unevenly distributed in the tissues of the human body. In hard tissues, divalent elements predominate: calcium (Ca) and magnesium (Mg), and in soft tissues - monovalent elements: potassium (K) and sodium (Na). In addition, a lot of phosphorus (P) accumulates in hard tissues, mainly in the form of phosphate salts. With a lack of minerals in food, these compounds are excreted from the body and normal metabolism is disturbed.
Mineral substances dissolved in blood plasma, intercellular and other body fluids create a certain osmotic pressure, which depends on the molar concentration of substances dissolved in the liquid. Salts increase osmotic pressure to a greater extent
degree than non-electrolytes at the same molar concentration, since salts dissociate to form ions. Osmotic pressure depends on the total number of non-dissociated molecules and ions. The osmotic pressure of blood, lymph and intercellular fluid of the human and animal body depends mainly on the sodium chloride (NaCl) dissolved in them.
Osmotic pressure in body fluids affects the distribution of water and solutes in tissues. In higher animals, the osmotic pressure is constant and amounts to 7.5 - 9.0 atm. Maintaining a constant osmotic pressure is ensured by the activity of the excretory organs, mainly the kidneys and sweat glands.
The entry of mineral salts into the blood leads to the entry of intercellular water into the blood, and therefore the concentration of salt in the blood decreases. The excess water and salt is then removed by the kidneys. A decrease in water in the tissues, reflexively acting on the nerve centers, causes thirst.
The normal vital activity of the human body can proceed only with certain properties of the intercellular and interstitial fluids. In this constancy of the environment, an important role is played by the acid-base balance, in which the reaction of blood, lymph and other body fluids is close to neutral. Acid-base balance is maintained by complex system regulators united into a single central nervous system. Such regulators are blood buffer systems, exchange of oxygen and carbon dioxide, carbon dioxide and chloride salts, excretory functions of the kidneys, lungs, sweat glands, etc.
In the process of complex transformation in the human body of foods rich in calcium, magnesium, sodium or potassium, alkaline compounds can be formed. Sources of alkali-forming elements include fruits, vegetables, legumes, milk and dairy products.
Other products, such as meat, fish, eggs, cheese, bread, cereals, pasta, in the process of transformation in the human body give acidic compounds.
The nature of nutrition can affect the shifts in the acid-base balance in the tissues of the human body. Acid-base balance often shifts > side of acidity. As a result of a sharp shift
allowable maximum standards for ash content, and when evaluating such products, they determine its amount.
Usually, two concepts are distinguished - “total (raw) ash” and “pure ash”. The concept of "total ash" means the sum of mineral elements or their oxides that are part of the chemical structure of food products, as well as introduced into the product during its production or "accidentally caught as impurities. "Pure ash" means the sum of mineral elements or their oxides without impurities .
The ash content of the product is determined by burning. To do this, the sample is first carefully burned, and then calcined to constant weight. An increased amount of ash against the norm indicates contamination of the product with sand, metal particles, and earth.
To determine "pure ash", the resulting ash is treated with 10% hydrochloric acid. In this case, “pure ash” dissolves in hydrochloric acid, and the residue will indicate the presence of foreign inorganic impurities in the product. So, in the case of poor washing of tomatoes before processing, or in potato starch, with insufficient washing of tubers, the atom product contains an increased amount of ash due to extraneous mineral impurities.
Calcium in the human body is found in bone tissue and teeth - about 99%. The rest of the calcium enters the blood in the form of ions and in the state associated with proteins and other compounds.
The daily requirement of an adult for calcium is 0.8-1.0 g. Pregnant and lactating women need increased amounts of calcium, up to 1.5-2 g per day, as well as children in whose bodies calcium is intensively used for bone formation. Calcium deficiency causes skeletal deformity, bone fragility and muscle atrophy in the body. Calcium is characterized by the feature that even with its lack in food, it continues to be excreted from the body in significant quantities.
Calcium is found in food products in the form of phosphate and oxalate chloride salts, as well as in combination with fatty acids, proteins, etc.
All calcium compounds, with the exception of CaC! a, are hardly soluble in water, and therefore are poorly absorbed
the human body. Insoluble calcium compounds partially pass from products into solution in the stomach under the action of of hydrochloric acid gastric juice. The absorption of calcium in food products by the human body depends to a large extent on the presence of phosphates, fats, magnesium compounds, etc. in food. Thus, the absorption of calcium is highest when the ratio of calcium and phosphorus I in food; 1.5 or 1: 2. The amount of phosphorus in food increased against the indicated ratios leads to a sharp decrease in the absorption of calcium. An excess of magnesium also has an adverse effect on the absorption of calcium by the human body. Sharp bad influence the absorption of calcium is exerted by calcium compounds with inositol-phosphoric acid, which is found in significant quantities in cereal grains and products of its processing.
Vitamin D plays a very important role in the absorption of calcium, which promotes the transition of calcium and phosphorus salts from the intestines into the blood and deposition in the bones in the form of calcium phosphate.
The content of calcium in some food products is as follows (mg%): in lean meat - 7; in eggs - 54; in milk - 118; in cheese - 930; in cottage cheese - 140; in oatmeal - 65; in wheat flour - 15; in rice - 9; in apples - 7; in oranges - 45; in walnuts -89; in beets - 29; in cauliflower - 89; in white cabbage - 45; in carrots - 56; in potatoes - 14. From the above data, it can be seen that the most important source of calcium for humans is dairy products. Calcium in dairy products, as well as vegetables and fruits, is an easily digestible compound.
Magnesium in the human body is 30-35 times less than calcium, but it is very important. Most magnesium is found in bone tissue. Magnesium plays a special role in chlorophyll-bearing plants, where it is part of the chlorophyll molecule. Like calcium, magnesium forms sparingly soluble compounds. Magnesium is especially difficult to assimilate in the presence of the LO$ ion.
The content of magnesium in some food products is as follows (mg%): in beans - 139; in oatmeal - 133; in peas - 107; in millet - 87; in wheat bread - 30; in potatoes - 28; in carrots - 21; in white cabbage -! Anna - 12; in apples - 8; in lemons - 7; in beef - 15; in eggs - 11; in milk - 12. Consequently, 2 * 35 magnesium is found in the largest quantities in grains and legumes.
The magnesium requirement for an adult is 400 mg per day.
Sodium is widely found in foods, especially animal products. The main source of sodium for the human body is NaCt (common salt). Sodium plays an important role in the processes of intracellular and intertissue metabolism. About 90% of the osmotic pressure of blood plasma depends on the content of NaCl in it. Typically, 3.3 g of sodium is dissolved in a liter of human blood plasma. NaC! It also plays an important role in regulating the body's water metabolism. Sodium ions cause swelling of tissue colloids and thereby contribute to the retention of bound water in the body. From the body of NaC! excreted mainly in urine and sweat. With increased work and consumption of liquids, a person loses up to 3-5 liters of sweat, which is 99.5% water. In the dry matter of sweat, the main part is NaGI.
Table salt, which enters the human body with food, replenishes the consumption of NaCl in the blood and is used to form hydrochloric acid in gastric juice, as well as to synthesize NaHCO3 by the pancreatic gland. The presence of NaHCO3 explains the alkaline reaction of pancreatic juice, which is necessary for the breakdown of food proteins by the enzyme trypsin.
The daily requirement of an adult for sodium is 4-6 g, which corresponds to 10-15 g of table salt. The usual diets of the population contain sufficient amounts of sodium, as table salt is added to food.
Potassium is constantly and in significant quantities present in food products, especially of plant origin. In the ashes of plants, the potassium content is sometimes more than 50% of its mass.
In the human body, potassium is involved in enzymatic reactions, the formation of buffer systems that prevent shifts in the reaction of the environment. Potassium reduces
water-retaining ability of proteins, reducing their hydro-(bility), and thereby promotes the excretion of water and sodium from the body. Therefore, potassium can be considered as some physiological sodium antagonist.
The daily requirement of an adult for potassium is 3-5 g.
Iron is widely distributed in nature. Generally, almost all natural foods contain iron, but in small amounts.
In human and animal organisms, iron is part of the most important organic compounds - blood hemoglobin, myoglobin, some enzymes - catalase, peroxidase, cytochrome oxidase, etc. Blood hemoglobin contains 2A, the body's iron. A significant amount of iron is found in the spleen and liver. Iron has the ability to accumulate in the body. Hemoglobin in the blood is destroyed during life, and the iron released in this case can be reused by the body to form hemoglobin.
Iron, which is part of fruits and vegetables, is well absorbed by the human body, while most of the iron in grain products is in an indigestible form for the body.
The daily requirement of an adult human gland is 15 mg.
l l o r is a part of natural foods in small quantities. Vegetable products contain little chlorine, while animal products contain slightly more. So, the chlorine content in beef is 76 mg%, in milk - 106, in eggs -
37106, in cheese - 880, in millet - 19, in potatoes - 54, in apples - 5 mg%.
The content of chlorine is significant in the blood and other body fluids, as well as in the skin, lungs, and kidneys. Chlorine in the body is in an ionized state in the form of anions of salts of sodium, potassium, calcium, magnesium, manganese. Compounds of chlorine in food products are highly soluble and easily absorbed in the human intestine. Chlorine anions, together with sodium cations, play an important role in creating and regulating the osmotic pressure of blood and other body fluids. Chlorine salts provide the formation of hydrochloric acid by the gastric mucosa.
The main need for chlorine is met by sodium chloride, which is added to food in the form of salt.
The total amount of sodium chloride in the human body is usually 10-15 g, but when eating food rich in chlorine salts, the chlorine content in the human body can reach a higher amount. The daily human need for chlorine is 5-7 g.
Sulfur is found in the highest quantities in cereal products, legumes, dairy products, meat, fish, and especially eggs. It is a part of almost all proteins of the human body and is especially abundant in amino acids - cystine, methionine. The exchange of sulfur in the body is mainly its transformation into the indicated amino acids. It is also involved in the formation of vitamin Bg (thiamine), insulin and some other compounds. There is a lot of sulfur in the proteinoids of supporting tissues, for example, in the keratin of hair, nails, etc.
When compounds are oxidized in the body, a significant part of sulfur is excreted in the urine in the form of sulfuric acid salts.
The daily requirement of an adult for sulfur with moderate work is about 1 g.
Iodine is contained in the body of a healthy person weighing 70 kg in an amount of approximately 25 mg. Half of this amount is in the thyroid gland, and the rest is in the muscle and bone tissues and in the blood. Iodine of inorganic compounds in the thyroid gland is replaced by organic compounds - thyroxine, di-iodothyroxine, triiodothyroxine. Iodine is quickly absorbed by the thyroid gland and a few hours after entering it, it turns into organic
connections. These compounds stimulate metabolic processes in the body. When an insufficient amount of iodine enters the body with food, the activity of the thyroid gland is disrupted and a serious disease called endemic goiter develops.
The largest amount of iodine is found in plant and animal products of coastal areas, where it is concentrated in sea water, air and soil of coastal areas. Little iodine accumulates in plants and animal organisms of mountainous or remote from the sea coast regions of iodine.
The content of iodine in grain products, vegetables, freshwater fish does not exceed 5-8 mcg per 100 g of raw product. Beef, eggs, butter, fruits are distinguished by a higher content of iodine. sea kale, marine fish and fish oil contain the highest amount of iodine. Feijoa fruits growing on the Black Sea coast of Georgia accumulate up to 390 micrograms of iodine per 100 g of fruit mass, which is much higher than the content of this element in other fruits and vegetables.
In areas where food products contain insufficient amounts of iodine, potassium iodide is added to table salt at the rate of 25 g K1 per ton of table salt. With a normal diet, a person consumes 200 micrograms of iodine per day with iodized salt. However, when storing iodized salt, iodine gradually disappears, so after 6 months iodized salt is sold as ordinary table salt.
The daily human need for iodine is 100-260 mcg.
Fluorine plays an important role in plastic processes during the formation of bone tissue and tooth enamel. The greatest amount of fluorine is concentrated in bones - 200-490 mg/kg and teeth - 240-560 mg/kg.
Water appears to be the main source of fluoride in the human body, with Doda's fluoride being better absorbed than food fluoride. The content of fluorine in drinking water ranges from 1 to 1.5 mg/l. The lack of fluorine in water often affects
39nne to the development of a disease of the teeth, known as caries. An excess of fluorine in water causes fluorosis, in which the normal structure of the teeth is disturbed, stains appear on the enamel and the fragility of the teeth increases. Children especially suffer from a lack or excess of fluorine.
The daily human need for fluorine has not yet been established. It is believed that the optimal amount of fluoride in drinking water for health should be 0.5-1.2 mg/l.
Copper in the animal body, along with iron, plays an important role in the processes of hematopoiesis, stimulates oxidative processes and is thus associated with iron metabolism. It is part of enzymes (lactase, ascorbate oxidase, cytochrome oxidase, etc.) as a metal component.
In plants, copper enhances oxidative processes, accelerates growth and increases the yield of many crops.
In those small quantities in which copper is found in natural products, it does not harm the human body. But elevated amounts of copper can cause poisoning. So, the simultaneous intake of 77-120 mg of copper can cause nausea, vomiting, and sometimes diarrhea. Therefore, the content of copper in food products is regulated by the current regulations of the Ministry of Health of the USSR. Per 1 kg of the product, depending on the content of solids in it, from 5 to 30 mg of copper is allowed. So, in concentrated tomato paste, the copper content should not exceed 30 mg / kg, in tomato puree - 15-20, in canned vegetables - 10, in jam and marmalade - 10, in fruit compotes - 5 mg / kg.
Copper can get into food products during their manufacture - from copper parts of equipment, when treating vineyards with pesticides containing copper, etc.
The daily requirement of an adult for copper is 2 mg.
Zinc is found in all tissues of animals and plants. With a lack of zinc in the organisms of young women,
In plants, their growth is delayed, and with its deficiency in the soil, diseases of many plants occur, which often leads to their death.
Zinc is part of a number of enzymes, and its role in the carbonic anhydrase enzyme molecule, which is involved in the binding and excretion of carbon dioxide from the animal body, is especially important. Zinc is essential for the normal function of pituitary, adrenal, and pancreatic hormones. It also has an effect on fat metabolism, increasing the breakdown of fats and preventing fatty liver.
Zinc in foods in high amounts can cause poisoning. Acidic and fatty foods dissolve metallic zinc, and therefore cooking or storing food in zinc equipment or utensils is unacceptable. Zinc poisoning is similar to copper poisoning, but more pronounced and is accompanied by burning and pain in the mouth and stomach, vomiting, diarrhea and heart weakness. Zinc utensils are allowed only for storing cold drinking water, since in this case the solubility of zinc is negligible.
The daily requirement of an adult for zinc is 10-15 mg. An increased need for zinc is observed during growth and puberty. With a normal diet, a person receives a sufficient amount of zinc from food.
Lead is found in animal and plant products in very small amounts. So, in apples, pears, grapes, strawberries, the lead content is about 0.1 mg per 1 kg of product, in milk - 0.8, in meat - 0.05, in sturgeon - 0.06 mg per 1 kg.
Lead is a toxic metal for humans, it has the ability to accumulate in the body, mainly in the liver, and cause severe chronic poisoning.
With daily use of 2-4 mg of lead with food, a sign of lead poisoning may be detected after a few months.
41 Food contamination with lead can be from utensils, solders, glazes, equipment, and lead-containing insecticides. Most often, lead poisoning occurs when food is stored in artisanal earthenware that is not well covered with lead glaze.
Due to the high toxicity, the content of lead in food products is not allowed.
Tin is found in food products in small quantities. Thus, 0.14 mg/kg of tin was found in the liver of a bull and a ram, 0.003 in the kidneys, 0.63 in the lungs, and 0.019 mg/kg in the brain.
Tin is not such a toxic metal as lead, zinc or copper, therefore it is allowed in limited quantities in the equipment of food enterprises, as well as for tinning the surface of tin, from which tin cans are prepared, protecting it from corrosion. However, often during long-term storage of canned food in cans, the mass of the product interacts with the tin coating of the tin, as a result of which tin salts of organic acids are formed. This process is especially active when there are products with high acidity in the tin - fruits, canned fish and vegetables in tomato sauce and others. During long-term storage, the content of tin in canned food can increase significantly. The content of tin increases especially rapidly in products that are in open metal cans coated with tin.
To enhance the protection of a tin can from corrosion, special acid-resistant varnishes or enamel are additionally applied to the surface of the tin, or a thin film of stable tin oxides is created on the surface of the tin.
Manganese is widely distributed in animal and vegetable products. It takes an active part in the formation of many enzymes, bone formation, hematopoiesis processes and stimulates growth. In plants, manganese enhances the process of photosynthesis and the formation of ascorbic acid.
Plant products are in most cases richer in manganese than animal products. So, the content of manganese in cereal products reaches 1-15 mg per 1 kg, in leaf
vegetables - 10-20, in fruits - 0.5-1, in milk - 0.02-0.03, in eggs - 0.1-0.2, in the liver of animals - 2.65-2.98 mg per 1 kg.
With a lack of manganese in the soil, plants become ill and develop poorly, the yield of fruits, vegetables and other crops decreases. The addition of microfertilizers containing manganese to the soil helps to increase the yield.
The daily requirement of an adult for manganese is 5-10 mg per day.
Radioactive isotopes are present in the human body, they continuously enter and exit the body. There is a balance between the intake of radioactive compounds into the body and their removal from the body. All food products contain radioactive isotopes of potassium (K40), carbon (C14), hydrogen (H3), and also radium with its decay products.
The highest concentration falls on potassium (K40). Isotopes participate in metabolism along with non-radioactive ones.
It is believed that during the nearest geological time there were no great changes in the intensity of radiation on Earth, therefore, in the animal and flora developed a kind of immunity to these levels of radiation. But living organisms are very sensitive to elevated concentrations. Small concentrations increase the growth of living organisms, large concentrations cause the appearance of active radicals, as a result of which there is a violation of the vital activity of individual organs and tissues, as well as the whole organism.
At atomic explosions radioactive isotopes fall on the Earth's surface, polluting the atmosphere, water, soil and plants. Through food, atmosphere and water, radioactive isotopes enter the human body.
It has been established that when food products are treated with radiation of radioactive isotopes, their shelf life increases, and the germination of potatoes is delayed. But usually, the irradiated food may develop a specific smell and taste, and it is possible that toxic substances may be formed. Long-term experiments are required to determine the safety of such products.
test questions
What chemical elements are macronutrients?
What are the functions of minerals in the human body?
What is the role of calcium in the human body?
What chemical elements are classified as trace elements and what are their functions in the human body?
What role does iron play in the human body and in what foods is it found?
What are the consequences of iodine deficiency in the body and how can this be avoided?
What types of technological processing of raw materials and food products contribute to the loss of minerals?
Give examples of the interaction of some microelements and vitamins.
What methods for determining the content of macro- and microelements do you know?
Kukushkin flax reproduces: by zoospores;
seeds under adverse conditions;
disputes; +
aplanospores.
- strawberry leaves:
unpaired pinnate;
ternary; +
ternary, single leaf;
complex unifolia. Worker bees are:
asexual individuals;
females with underdeveloped reproductive organs; +
males with underdeveloped reproductive organs;
males and females with normally developed reproductive organs, but temporarily not breeding. Digestion in coral polyps:
only cavity;
only intracellular;
abdominal and intracellular; +
cavity, intracellular and external. Pteropod molluscs that have the ability to glow in the dark can be part of:
benthos;
neuston;
phytoplankton;
zooplankton. + The blowfly development cycle was first described by:
Anton Levenguk;
Francesco Redi; +
Henri Fabre;
Louis Pasteur. Butterfly caterpillars have:
three pairs of pectoral legs;
three pairs of thoracic legs and five pairs of ventral false legs; +
eight pairs of false legs;
limbs are missing. The circulatory system of the lancelet:
open;
closed, there is one circle of blood circulation; +
closed, there are two circles of blood circulation;
missing. Choose the correct sentences:
- Choose the correct sentences:
- In cyclostomes, the digestive tract has:
the shape of a straight tube;
hepatic outgrowth;
pyloric outgrowths;
spiral valve. + From fish of the Sturgeon order is not passing view:
beluga;
stellate sturgeon;
sterlet; +
sturgeon. Salivary glands during the evolution of vertebrates first appear in:
lungfish;
amphibians; +
reptiles;
mammals. Of the fish of the Cod order, it lives and spawns only in fresh water:
cod;
haddock;
burbot; +
pollock. The origin of the bird's wing from the free forelimb characteristic of four-legged vertebrates is clearly illustrated by the example of chicks:
ostrich;
kiwi;
hoatzin; +
penguin. On the aerodynamic properties of a bird in flight do not affect feathers:
flywheels;
downy; +
steering;
contour. Among birds, stereoscopic vision is most developed in species:
insectivores;
granivorous;
carnivorous; +
planktivorous.
The glycocalyx of animal cells form:
proteins and lipids;
proteins and nucleotides;
proteins and carbohydrates; +
carbohydrates and nucleotides.
The process by which the dysenteric amoeba engulfs red blood cells:
osmosis;
pinocytosis;
phagocytosis; +
facilitated diffusion.
Pithecanthropus remains were first discovered in:
South Africa;
Australia;
Central Asia;
South-East Asia. +
The most ancient of the named fossil ancestors of man is:
Neanderthal;
Pithecanthropus;
Australopithecus; +
Cro-Magnon.
Organelles found in cells of both prokaryotes and eukaryotes:
endoplasmic reticulum;
mitochondria;
lysosomes;
ribosomes. +
The main components of eukaryotic nuclear chromatin are:
DNA and RNA;
RNA and proteins;
DNA and proteins; +
DNA and lipids. microtubules do not provide:
maintaining the shape of the cell;
change in the shape of the cell; +
movement of organelles;
movement of chromosomes during cell division. Cellular proteins destined for secretion are sorted and packaged into:
lysosomes;
endosomes;
endoplasmic reticulum;
trans Golgi networks. +
The location of the ATP synthetase enzyme in mitochondria is:
matrix;
intermembrane space;
outer membrane;
inner membrane. +
Oxidation of organic compounds to CO 2 in mitochondria occurs:
in the matrix; +
in the intermembrane space;
on the outer membrane;
on the inner membrane.
The anticodon contains:
one nucleotide;
two nucleotides;
three nucleotides; +
four nucleotides.
The final electron acceptor in cellular respiration is:
NADH;
water;
oxygen; +
ATP.
A property of the genetic code that increases the reliability of storage and transmission of genetic information:
triplet;
universality;
redundancy; +
lack of punctuation marks.
Magnesium ions are part of:
hemoglobin;
insulin;
chlorophyll; +
thyroxine. RNA molecules capable of exhibiting catalytic activity are called:
ribonucleases;
ribosomes;
ribozymes; +
ribonucleotides. Macroergic compounds are called:
characterized by the presence of covalent bonds with high energy;
in the destruction of certain bonds in which a large amount of free energy is released; +
the synthesis of which occurs with the expenditure of a large amount of energy;
which give off a lot of heat when burned.
In the process of photosynthesis, the source of oxygen as a by-product is:
ribulose bisphosphate;
glucose;
water; +
carbon dioxide.
The development of nitrifying bacteria leads to:
acidification of the environment; +
alkalization of the environment;
neutralization of the environment;
does not affect the pH of the medium.
Acidophilus is formed as a result of the fermentation of milk:
lactic acid bacteria; +
yeast;
mixed culture of lactic acid bacteria and yeast;
mixed culture of lactic acid and propionic acid bacteria.
Of these diseases, it is caused by a virus:
cholera;
smallpox; +
plague;
malaria.
Of the plant cell components, the tobacco mosaic virus infects:
mitochondria;
chloroplasts; +
nucleus;
vacuoles.