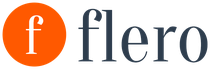What heats up faster on the stove - a kettle or a bucket of water? The answer is obvious - a kettle. Then the second question is why?
The answer is no less obvious - because the mass of water in the kettle is less. Fine. And now you can do the most real physical experience yourself at home. To do this, you will need two identical small saucepans, an equal amount of water and vegetable oil, for example, half a liter each and a stove. Put pots of oil and water on the same fire. And now just watch what will heat up faster. If there is a thermometer for liquids, you can use it, if not, you can just try the temperature from time to time with your finger, just be careful not to burn yourself. In any case, you will soon see that the oil heats up significantly faster than water. And one more question, which can also be implemented in the form of experience. Which boils faster - warm water or cold? Everything is obvious again - the warm one will be the first to finish. Why all these strange questions and experiences? In order to determine the physical quantity called "the amount of heat."
Quantity of heat
The amount of heat is the energy that the body loses or gains during heat transfer. This is clear from the name. When cooling, the body will lose a certain amount of heat, and when heated, it will absorb. And the answers to our questions showed us what does the amount of heat depend on? First, the greater the mass of the body, the greater the amount of heat that must be expended to change its temperature by one degree. Secondly, the amount of heat necessary to heat a body depends on the substance of which it is composed, that is, on the kind of substance. And thirdly, the difference in body temperature before and after heat transfer is also important for our calculations. Based on the foregoing, we can determine the amount of heat by the formula:
where Q is the amount of heat,
m - body weight,
(t_2-t_1) - the difference between the initial and final body temperatures,
c - specific heat capacity of the substance, is found from the relevant tables.
Using this formula, you can calculate the amount of heat that is necessary to heat any body or that this body will release when it cools.
The amount of heat is measured in joules (1 J), like any other form of energy. However, this value was introduced not so long ago, and people began to measure the amount of heat much earlier. And they used a unit that is widely used in our time - a calorie (1 cal). 1 calorie is the amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius. Guided by these data, lovers of counting calories in the food they eat can, for the sake of interest, calculate how many liters of water can be boiled with the energy that they consume with food during the day.
730. Why is water used to cool some mechanisms?
Water has great specific heat, which contributes to good heat dissipation from the mechanism.
731. In what case should more energy be expended: for heating one liter of water by 1 °C or for heating one hundred grams of water by 1 °C?
To heat a liter of water, since the larger the mass, the more energy needs to be expended.
732. Cupronickel and silver forks of the same mass were dipped into hot water. Do they receive the same amount of heat from water?
A cupronickel fork will receive more heat, because the specific heat of cupronickel is greater than that of silver.
733. A piece of lead and a piece of cast iron of the same mass were hit three times with a sledgehammer. Which part got hotter?
Lead will heat up more because its specific heat capacity is less than cast iron, and less energy is needed to heat the lead.
734. One flask contains water, the other contains kerosene of the same mass and temperature. An equally heated iron cube was thrown into each flask. What will heat up to a higher temperature - water or kerosene?
Kerosene.
735. Why are temperature fluctuations less sharp in winter and summer in cities on the seashore than in cities located inland?
Water heats up and cools down more slowly than air. In winter, it cools down and moves warm air masses on land, making the climate on the coast warmer.
736. Specific heat aluminum is 920 J/kg °C. What does this mean?
This means that it takes 920 J to heat 1 kg of aluminum by 1 °C.
737. Aluminum and copper bars of the same mass of 1 kg are cooled by 1 °C. How much will the internal energy of each block change? Which bar will change more and by how much?
738. What amount of heat is needed to heat a kilogram iron billet by 45 °C?
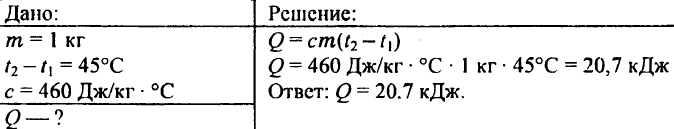
739. How much heat is required to heat 0.25 kg of water from 30°C to 50°C?

740. How will the internal energy of two liters of water change when heated by 5 °C?

741. How much heat is needed to heat 5 g of water from 20 °C to 30 °C?

742. What amount of heat is needed to heat an aluminum ball weighing 0.03 kg by 72 °C?

743. Calculate the amount of heat required to heat 15 kg of copper by 80 °C.

744. Calculate the amount of heat required to heat 5 kg of copper from 10 °C to 200 °C.
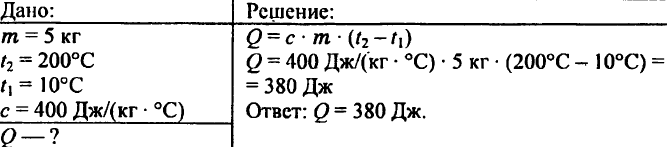
745. What amount of heat is required to heat 0.2 kg of water from 15 °C to 20 °C?

746. Water weighing 0.3 kg has cooled down by 20 °C. By how much is the internal energy of water reduced?

747. How much heat is needed to heat 0.4 kg of water at a temperature of 20 °C to a temperature of 30 °C?

748. How much heat is spent on heating 2.5 kg of water by 20 °C?
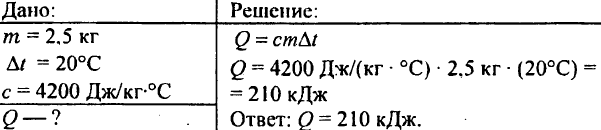
749. How much heat was released when 250 g of water cooled from 90 °C to 40 °C?

750. What amount of heat is required to heat 0.015 liters of water by 1 °C?

751. Calculate the amount of heat required to heat a pond with a volume of 300 m3 by 10 °C?

752. How much heat must be imparted to 1 kg of water in order to raise its temperature from 30°C to 40°C?
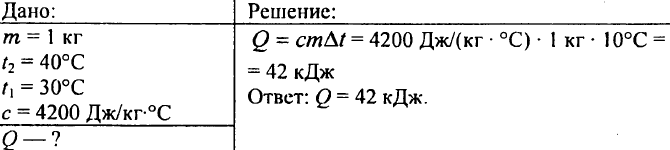
753. Water with a volume of 10 liters has cooled down from a temperature of 100 °C to a temperature of 40 °C. How much heat is released in this case?

754. Calculate the amount of heat required to heat 1 m3 of sand by 60 °C.

755. Air volume 60 m3, specific heat capacity 1000 J/kg °C, air density 1.29 kg/m3. How much heat is needed to raise it to 22°C?

756. Water was heated by 10 ° C, spending 4.20 103 J of heat. Determine the amount of water.

757. Water weighing 0.5 kg reported 20.95 kJ of heat. What was the temperature of the water if the initial temperature of the water was 20°C?
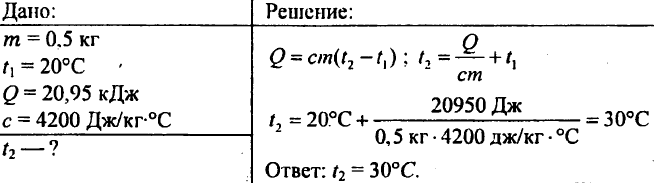
758. 8 kg of water at 10 °C is poured into a copper saucepan weighing 2.5 kg. How much heat is needed to bring the water to a boil in a saucepan?

759. A liter of water at a temperature of 15 °C is poured into a copper ladle weighing 300 g. How much heat is needed to heat the water in the ladle by 85 °C?

760. A piece of heated granite weighing 3 kg is placed in water. Granite transfers 12.6 kJ of heat to water, cooling by 10 °C. What is the specific heat capacity of the stone?

761. Hot water at 50°C was added to 5 kg of water at 12°C, obtaining a mixture with a temperature of 30°C. How much water was added?

762. Water at 20°C was added to 3 liters of water at 60°C to obtain water at 40°C. How much water was added?

763. What will be the temperature of the mixture if 600 g of water at 80 °C are mixed with 200 g of water at 20 °C?

764. A liter of water at 90°C was poured into water at 10°C, and the temperature of the water became 60°C. How much was cold water?

765. Determine how much to pour into a vessel hot water, heated to 60 ° C, if the vessel already contains 20 liters of cold water at a temperature of 15 ° C; the temperature of the mixture should be 40 °C.

766. Determine how much heat is required to heat 425 g of water by 20 °C.

767. How many degrees will 5 kg of water heat up if the water receives 167.2 kJ?

768. How much heat is required to heat m grams of water at a temperature t1 to a temperature t2?
769. 2 kg of water is poured into a calorimeter at a temperature of 15 °C. To what temperature will the water of the calorimeter heat up if a brass weight of 500 g heated to 100 °C is lowered into it? The specific heat capacity of brass is 0.37 kJ/(kg °C).
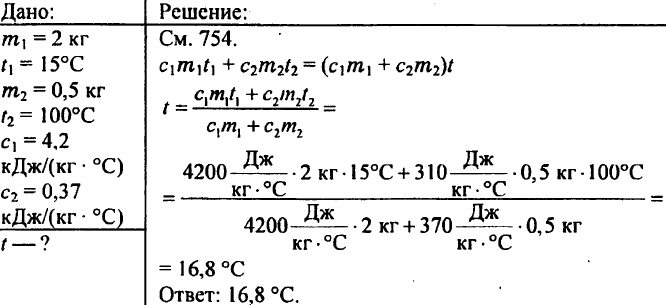
770. There are pieces of copper, tin and aluminum of the same volume. Which of these pieces has the largest and which the smallest heat capacity?

771. 450 g of water, the temperature of which is 20 °C, was poured into the calorimeter. When 200 g of iron filings heated to 100°C were immersed in this water, the temperature of the water became 24°C. Determine the specific heat capacity of sawdust.
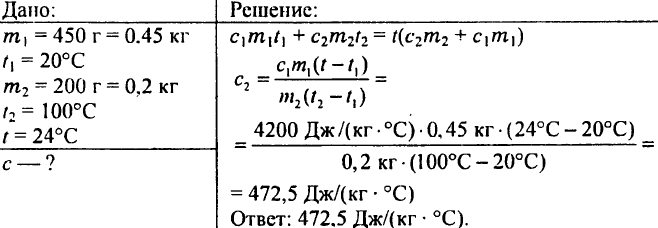
772. A copper calorimeter weighing 100 g holds 738 g of water, the temperature of which is 15 °C. 200 g of copper was lowered into this calorimeter at a temperature of 100 °C, after which the temperature of the calorimeter rose to 17 °C. What is the specific heat capacity of copper?

773. A steel ball weighing 10 g is taken out of the furnace and lowered into water at a temperature of 10 °C. The water temperature rose to 25°C. What was the temperature of the ball in the oven if the mass of water is 50 g? The specific heat capacity of steel is 0.5 kJ/(kg °C).

777. 50 g of water at 19 °C are poured into water weighing 150 g at a temperature of 35 °C. What is the temperature of the mixture?

778. Water weighing 5 kg at 90 °C was poured into a cast-iron kettle weighing 2 kg at a temperature of 10 °C. What was the temperature of the water?

779. A steel chisel weighing 2 kg was heated to a temperature of 800 °C and then lowered into a vessel containing 15 liters of water at a temperature of 10 °C. To what temperature will the water in the vessel be heated?
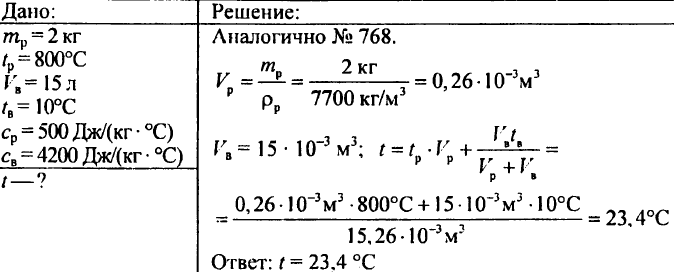
(Indication. To solve this problem, it is necessary to create an equation in which the desired temperature of the water in the vessel after the cutter is lowered is taken as the unknown.)
780. What temperature will water get if you mix 0.02 kg of water at 15 °C, 0.03 kg of water at 25 °C, and 0.01 kg of water at 60 °C?

781. Heating a well-ventilated class requires an amount of heat of 4.19 MJ per hour. Water enters the heating radiators at 80°C and exits at 72°C. How much water should be supplied to the radiators every hour?

782. Lead weighing 0.1 kg at a temperature of 100 °C was immersed in an aluminum calorimeter weighing 0.04 kg containing 0.24 kg of water at a temperature of 15 °C. After that, the temperature of 16 °C was established in the calorimeter. What is the specific heat capacity of lead?
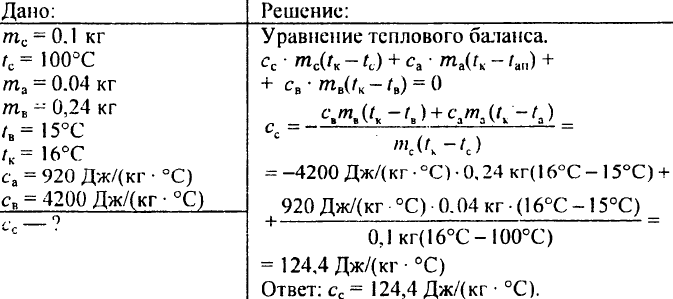
Heat capacity is the amount of heat absorbed by the body when heated by 1 degree.
The heat capacity of a body is indicated by capital letters Latin letter With.
What determines the heat capacity of a body? First of all, from its mass. It is clear that heating, for example, 1 kilogram of water will require more heat than heating 200 grams.
What about the kind of substance? Let's do an experiment. Let's take two identical vessels and, pouring water weighing 400 g into one of them, and vegetable oil weighing 400 g into the other, we will begin to heat them with the help of identical burners. By observing the readings of thermometers, we will see that the oil heats up quickly. To heat water and oil to the same temperature, the water must be heated longer. But the longer we heat the water, the more heat it receives from the burner.
Thus, to heat the same mass of different substances to the same temperature, different amounts of heat are required. The amount of heat required to heat a body and, consequently, its heat capacity depend on the kind of substance of which this body is composed.
So, for example, to increase the temperature of water with a mass of 1 kg by 1 ° C, an amount of heat equal to 4200 J is required, and to heat the same mass of sunflower oil by 1 ° C, an amount of heat equal to 1700 J is required.
The physical quantity showing how much heat is required to heat 1 kg of a substance by 1 ºС is called specific heat this substance.
Each substance has its own specific heat capacity, which is denoted by the Latin letter c and is measured in joules per kilogram-degree (J / (kg ° C)).
The specific heat capacity of the same substance in different aggregate states (solid, liquid and gaseous) is different. For example, the specific heat capacity of water is 4200 J/(kg ºС), and the specific heat capacity of ice is 2100 J/(kg ºС); aluminum in the solid state has a specific heat capacity of 920 J / (kg - ° C), and in the liquid state - 1080 J / (kg - ° C).
Note that water has a very high specific heat capacity. Therefore, the water in the seas and oceans, heating up in summer, absorbs a large amount of heat from the air. Due to this, in those places that are located near large bodies of water, summer is not as hot as in places far from water.
Calculation of the amount of heat required to heat the body or released by it during cooling.
From the foregoing, it is clear that the amount of heat necessary to heat the body depends on the type of substance of which the body consists (i.e., its specific heat capacity) and on the mass of the body. It is also clear that the amount of heat depends on how many degrees we are going to increase the temperature of the body.
So, to determine the amount of heat required to heat the body or released by it during cooling, you need to multiply the specific heat of the body by its mass and by the difference between its final and initial temperatures:
Q= cm (t 2 -t 1),
where Q- quantity of heat, c- specific heat capacity, m- body mass, t1- initial temperature, t2- final temperature.
When the body is heated t2> t1 and hence Q >0 . When the body is cooled t 2and< t1 and hence Q< 0 .
If the heat capacity of the whole body is known With, Q is determined by the formula: Q \u003d C (t 2 - t1).
22) Melting: definition, calculation of the amount of heat for melting or solidification, specific heat of melting, graph of t 0 (Q).
Thermodynamics
A branch of molecular physics that studies the transfer of energy, the patterns of transformation of some types of energy into others. Unlike the molecular-kinetic theory, thermodynamics does not take into account the internal structure of substances and microparameters.
Thermodynamic system
This is a collection of bodies that exchange energy (in the form of work or heat) with each other or with environment. For example, the water in the teapot cools down, the exchange of heat of the water with the teapot and of the teapot with the environment takes place. Cylinder with gas under the piston: the piston performs work, as a result of which the gas receives energy and its macro parameters change.
Quantity of heat
This is energy, which is received or given by the system in the process of heat exchange. Denoted by the symbol Q, measured, like any energy, in Joules.
As a result of various heat transfer processes, the energy that is transferred is determined in its own way.
Heating and cooling
This process is characterized by a change in the temperature of the system. The amount of heat is determined by the formula

The specific heat capacity of a substance with measured by the amount of heat required to heat up mass units of this substance by 1K. Heating 1 kg of glass or 1 kg of water requires a different amount of energy. Specific heat capacity is a known value already calculated for all substances, see the value in physical tables.
Heat capacity of substance C- this is the amount of heat that is necessary to heat the body without taking into account its mass by 1K.


Melting and crystallization
Melting is the transition of a substance from a solid to a liquid state. The reverse transition is called crystallization.
Energy spent on destruction crystal lattice substances, is determined by the formula


Specific heat melting value known for each substance, see the value in physical tables.
Vaporization (evaporation or boiling) and condensation
Vaporization is the transition of a substance from a liquid (solid) state to a gaseous state. The reverse process is called condensation.


The specific heat of vaporization is a known value for each substance, see the value in the physical tables.
Combustion
The amount of heat released when a substance burns


The specific heat of combustion is a known value for each substance, see the value in the physical tables.
For a closed and adiabatically isolated system of bodies, the heat balance equation is satisfied. The algebraic sum of the amounts of heat given and received by all bodies participating in heat exchange is equal to zero:
Q 1 +Q 2 +...+Q n =0
23) The structure of liquids. surface layer. Surface tension force: examples of manifestation, calculation, surface tension coefficient.
From time to time, any molecule can move to an adjacent vacancy. Such jumps in liquids occur quite often; therefore, the molecules are not tied to certain centers, as in crystals, and can move throughout the entire volume of the liquid. This explains the fluidity of liquids. Due to the strong interaction between closely spaced molecules, they can form local (unstable) ordered groups containing several molecules. This phenomenon is called short-range order(Fig. 3.5.1).
The coefficient β is called temperature coefficient of volume expansion . This coefficient for liquids is ten times greater than for solids. For water, for example, at a temperature of 20 ° C, β in ≈ 2 10 - 4 K - 1, for steel β st ≈ 3.6 10 - 5 K - 1, for quartz glass β kv ≈ 9 10 - 6 K - one .
The thermal expansion of water has an interesting and important anomaly for life on Earth. At temperatures below 4 °C, water expands with decreasing temperature (β< 0). Максимум плотности ρ в = 10 3 кг/м 3 вода имеет при температуре 4 °С.
When water freezes, it expands, so the ice remains floating on the surface of the freezing body of water. The temperature of freezing water under ice is 0°C. In denser layers of water near the bottom of the reservoir, the temperature is about 4 °C. Thanks to this, life can exist in the water of freezing reservoirs.
Most interesting feature liquids is the presence free surface . Liquid, unlike gases, does not fill the entire volume of the vessel into which it is poured. An interface is formed between the liquid and the gas (or vapor), which is in special conditions compared to the rest of the liquid mass. It should be borne in mind that, due to the extremely low compressibility, the presence of a more densely packed surface layer does not lead to any noticeable change in the volume of the liquid . If the molecule moves from the surface into the liquid, the forces of intermolecular interaction will do positive work. On the contrary, in order to pull a certain number of molecules from the depth of the liquid to the surface (i.e., increase the surface area of the liquid), external forces must do a positive work Δ A external, proportional to the change Δ S surface area:
It is known from mechanics that the equilibrium states of a system correspond to the minimum value of its potential energy. It follows that the free surface of the liquid tends to reduce its area. For this reason, a free drop of liquid takes on a spherical shape. The fluid behaves as if forces are acting tangentially to its surface, reducing (contracting) this surface. These forces are called surface tension forces .
The presence of surface tension forces makes the liquid surface look like an elastic stretched film, with the only difference that the elastic forces in the film depend on its surface area (i.e., on how the film is deformed), and the surface tension forces do not depend on the surface area of the liquid.
Some liquids, such as soapy water, have the ability to form thin films. All well-known soap bubbles have the correct spherical shape - this also manifests the action of surface tension forces. If a wire frame is lowered into the soapy solution, one of the sides of which is movable, then the whole of it will be covered with a film of liquid (Fig. 3.5.3).
Surface tension forces tend to shorten the surface of the film. To balance the moving side of the frame, an external force must be applied to it. If, under the action of the force, the crossbar moves by Δ x, then the work Δ A ext = F ext Δ x = Δ Ep = σΔ S, where ∆ S = 2LΔ x is the increment in the surface area of both sides of the soap film. Since the moduli of forces and are the same, we can write:
|
Thus, the surface tension coefficient σ can be defined as modulus of the surface tension force acting per unit length of the line bounding the surface.
Due to the action of surface tension forces in liquid droplets and inside soap bubbles overpressure occurs Δ p. If we mentally cut a spherical drop of radius R into two halves, then each of them must be in equilibrium under the action of surface tension forces applied to the boundary of the cut with a length of 2π R and strength overpressure acting on the area π R 2 sections (Fig. 3.5.4). The equilibrium condition is written as
If these forces are greater than the forces of interaction between the molecules of the liquid itself, then the liquid wets the surface of a solid body. In this case, the liquid approaches the surface of the solid body at some acute angle θ, which is characteristic of the given liquid-solid pair. The angle θ is called contact angle . If the interaction forces between liquid molecules exceed the forces of their interaction with solid molecules, then the contact angle θ turns out to be obtuse (Fig. 3.5.5). In this case, the liquid is said to does not wet the surface of a solid body. At complete wettingθ = 0, at complete non-wettingθ = 180°.
capillary phenomena called the rise or fall of fluid in small diameter tubes - capillaries. Wetting liquids rise through the capillaries, non-wetting liquids descend.
On fig. 3.5.6 shows a capillary tube of a certain radius r lowered by the lower end into a wetting liquid of density ρ. The upper end of the capillary is open. The rise of the liquid in the capillary continues until the force of gravity acting on the liquid column in the capillary becomes equal in absolute value to the resulting F n surface tension forces acting along the boundary of contact of the liquid with the surface of the capillary: F t = F n, where F t = mg = ρ hπ r 2 g, F n = σ2π r cos θ.
This implies:
With complete nonwetting, θ = 180°, cos θ = –1 and, therefore, h < 0. Уровень несмачивающей жидкости в капилляре опускается ниже уровня жидкости в сосуде, в которую опущен капилляр.
Water almost completely wets the clean glass surface. Conversely, mercury does not completely wet the glass surface. Therefore, the level of mercury in the glass capillary falls below the level in the vessel.
24) Vaporization: definition, types (evaporation, boiling), calculation of the amount of heat for vaporization and condensation, specific heat of vaporization.
Evaporation and condensation. Explanation of the phenomenon of evaporation based on ideas about the molecular structure of matter. Specific heat of vaporization. Her units.
The phenomenon of liquid turning into vapor is called vaporization.
Evaporation - the process of vaporization occurring from an open surface.
Liquid molecules move at different speeds. If any molecule is at the surface of the liquid, it can overcome the attraction of neighboring molecules and fly out of the liquid. The escaping molecules form vapor. The velocities of the remaining liquid molecules change upon collision. In this case, some molecules acquire a speed sufficient to fly out of the liquid. This process continues, so liquids evaporate slowly.
*Evaporation rate depends on the type of liquid. Those liquids evaporate faster, in which the molecules are attracted with less force.
*Evaporation can occur at any temperature. But at higher temperatures, evaporation is faster .
*Evaporation rate depends on its surface area.
*With wind (air flow), evaporation occurs faster.
During evaporation, the internal energy decreases, because. during evaporation, fast molecules leave the liquid, therefore, the average speed of the remaining molecules decreases. This means that if there is no influx of energy from outside, then the temperature of the liquid decreases.
 The phenomenon of the transformation of vapor into liquid is called condensation.
It is accompanied by the release of energy.
The phenomenon of the transformation of vapor into liquid is called condensation.
It is accompanied by the release of energy.
Vapor condensation explains the formation of clouds. Water vapor rising above the ground forms clouds in the upper cold layers of air, which consist of tiny drops of water.
Specific heat of vaporization - physical. a quantity indicating how much heat is needed to turn a liquid of mass 1 kg into vapor without changing the temperature.
Oud. heat of vaporization denoted by the letter L and is measured in J / kg
Oud. heat of vaporization of water: L=2.3×10 6 J/kg, alcohol L=0.9×10 6
The amount of heat required to turn a liquid into steam: Q = Lm
The internal energy of the body can change due to the work of external forces. To characterize the change in internal energy during heat transfer, a quantity called the amount of heat and denoted by Q is introduced.
In the international system, the unit of the amount of heat, as well as work and energy, is the joule: = = = 1 J.
In practice, an off-system unit of the amount of heat is sometimes used - a calorie. 1 cal. = 4.2 J.
It should be noted that the term "quantity of heat" is unfortunate. It was introduced at a time when it was believed that bodies contained some weightless, elusive liquid - caloric. The process of heat transfer allegedly consists in the fact that caloric, pouring from one body into another, carries with it a certain amount of heat. Now, knowing the basics of the molecular-kinetic theory of the structure of matter, we understand that there is no caloric in bodies, the mechanism for changing the internal energy of a body is different. However, the power of tradition is great and we continue to use the term, introduced on the basis of incorrect ideas about the nature of heat. At the same time, understanding the nature of heat transfer, one should not completely ignore misconceptions about it. On the contrary, by drawing an analogy between the flow of heat and the flow of a hypothetical fluid of caloric, the amount of heat and the amount of caloric, it is possible, when solving some classes of problems, to visualize the ongoing processes and solve problems correctly. In the end, the correct equations describing the processes of heat transfer were obtained at one time on the basis of incorrect ideas about caloric as a heat carrier.
Let us consider in more detail the processes that can occur as a result of heat transfer.
Pour some water into a test tube and close it with a cork. Hang the test tube to a rod fixed in a tripod and bring an open flame under it. From the flame, the test tube receives a certain amount of heat and the temperature of the liquid in it rises. As the temperature rises, the internal energy of the liquid increases. There is an intensive process of its vaporization. The expanding liquid vapors do mechanical work to push the stopper out of the tube.
Let's conduct another experiment with a model of a cannon made from a piece of brass tube, which is mounted on a trolley. On one side, the tube is tightly closed with an ebonite plug, through which a pin is passed. Wires are soldered to the stud and tube, ending in terminals that can be energized from the lighting network. The gun model is thus a kind of electric boiler.
 |
Pour some water into the cannon barrel and close the tube with a rubber stopper. Connect the gun to a power source. An electric current passing through water heats it up. Water boils, which leads to its intense vaporization. The pressure of water vapor increases and, finally, they do the work of pushing the cork out of the gun barrel.
The gun, due to recoil, rolls back in the direction opposite to the cork launch.
Both experiences are united by the following circumstances. During the heating of the liquid different ways, the temperature of the liquid and, accordingly, its internal energy increased. In order for the liquid to boil and evaporate intensively, it was necessary to continue heating it.
The vapors of the liquid, due to their internal energy, performed mechanical work.
 |
We investigate the dependence of the amount of heat necessary to heat the body on its mass, temperature changes and the type of substance. To study these dependencies, we will use water and oil. (To measure the temperature in the experiment, an electric thermometer is used, made of a thermocouple connected to a mirror galvanometer. One thermocouple junction is lowered into a vessel with cold water to ensure its temperature is constant. The other thermocouple junction measures the temperature of the liquid under study).
The experience consists of three series. In the first series, for a constant mass of a particular liquid (in our case, water), the dependence of the amount of heat required to heat it on temperature change is studied. We will judge the amount of heat received by the liquid from the heater (electric stove) by the heating time, assuming that there is a direct relationship between them. proportional dependence. In order for the result of the experiment to correspond to this assumption, it is necessary to ensure a steady flow of heat from the electric stove to the heated body. To do this, the electric stove was connected to the network in advance, so that by the beginning of the experiment the temperature of its surface would cease to change. For more uniform heating of the liquid during the experiment, we will stir it with the help of the thermocouple itself. We will record the readings of the thermometer at regular intervals until the light spot reaches the edge of the scale.
Let us conclude: there is a direct proportional relationship between the amount of heat required to heat a body and a change in its temperature.
In the second series of experiments, we will compare the amount of heat required to heat the same liquids of different masses when their temperature changes by the same amount.
For the convenience of comparing the obtained values, the mass of water for the second experiment will be taken two times less than in the first experiment.
Again, we will record the thermometer readings at regular intervals.
Comparing the results of the first and second experiments, we can draw the following conclusions.
In the third series of experiments, we will compare the amount of heat required to heat equal masses of different liquids when their temperature changes by the same amount.
We will heat oil on an electric stove, the mass of which is equal to the mass of water in the first experiment. We will record the thermometer readings at regular intervals.
The result of the experiment confirms the conclusion that the amount of heat necessary to heat the body is directly proportional to the change in its temperature and, in addition, indicates the dependence of this amount of heat on the type of substance.
Since oil was used in the experiment, the density of which is less than the density of water, and a smaller amount of heat was required to heat the oil to a certain temperature than to heat water, it can be assumed that the amount of heat required to heat the body depends on its density.
To test this assumption, we will simultaneously heat identical masses of water, paraffin and copper on a heater of constant power.
After the same time, the temperature of copper is about 10 times, and paraffin is about 2 times higher than the temperature of water.
But copper has a greater and paraffin less density than water.
Experience shows that the quantity that characterizes the rate of change in the temperature of the substances from which the bodies involved in heat exchange are made is not the density. This quantity is called the specific heat capacity of the substance and is denoted by the letter c.
 |
A special device is used to compare the specific heat capacities of various substances. The device consists of racks in which a thin paraffin plate and a bar with rods passed through it are attached. Aluminum, steel and brass cylinders of equal mass are fixed at the ends of the rods.
We heat the cylinders to the same temperature by immersing them in a vessel of water standing on a hot electric stove. Let's fix the hot cylinders on the racks and release them from the fasteners. The cylinders simultaneously touch the paraffin plate and, melting the paraffin, begin to sink into it. The depth of immersion of cylinders of the same mass into a paraffin plate, when their temperature changes by the same amount, turns out to be different.
Experience shows that the specific heat capacities of aluminum, steel and brass are different.
Having done the corresponding experiments with the melting of solids, the vaporization of liquids, and the combustion of fuel, we obtain the following quantitative dependences.
To obtain units of specific quantities, they must be expressed from the corresponding formulas and the units of heat - 1 J, mass - 1 kg, and for specific heat - and 1 K should be substituted into the resulting expressions.
We get units: specific heat capacity - 1 J / kg K, other specific heats: 1 J / kg.
The process of transferring energy from one body to another without doing work is called heat exchange or heat transfer. Heat transfer occurs between bodies that have different temperatures. When contact is established between bodies with different temperatures, a part of the internal energy is transferred from a body with a higher temperature to a body with a lower temperature. The energy transferred to the body as a result of heat transfer is called amount of heat.
Specific heat capacity of a substance:
If the heat transfer process is not accompanied by work, then, based on the first law of thermodynamics, the amount of heat is equal to the change in the internal energy of the body: .
The average energy of the random translational motion of molecules is proportional to the absolute temperature. The change in the internal energy of a body is equal to the algebraic sum of the changes in the energy of all atoms or molecules, the number of which is proportional to the mass of the body, so the change in internal energy and, consequently, the amount of heat is proportional to the mass and temperature change:


The proportionality factor in this equation is called specific heat capacity of a substance. The specific heat capacity indicates how much heat is needed to raise the temperature of 1 kg of a substance by 1 K.
Work in thermodynamics:
In mechanics, work is defined as the product of the modules of force and displacement and the cosine of the angle between them. Work is done when a force acts on a moving body and is equal to the change in its kinetic energy.
In thermodynamics, the motion of a body as a whole is not considered; we are talking about the movement of parts of a macroscopic body relative to each other. As a result, the volume of the body changes, and its velocity remains equal to zero. Work in thermodynamics is defined in the same way as in mechanics, but it is equal to the change not in the kinetic energy of the body, but in its internal energy.
When work is done (compression or expansion), the internal energy of the gas changes. The reason for this is as follows: during elastic collisions of gas molecules with a moving piston, their kinetic energy changes.
Let us calculate the work of the gas during expansion. The gas acts on the piston with a force  , where
, where  is the pressure of the gas, and
is the pressure of the gas, and  - surface area
- surface area  piston. As the gas expands, the piston moves in the direction of the force
piston. As the gas expands, the piston moves in the direction of the force  for a short distance
for a short distance  . If the distance is small, then the gas pressure can be considered constant. The work of the gas is:
. If the distance is small, then the gas pressure can be considered constant. The work of the gas is:
Where  - change in gas volume.
- change in gas volume.
In the process of expanding the gas, it does positive work, since the direction of force and displacement coincide. In the process of expansion, the gas gives off energy to the surrounding bodies.
The work done by external bodies on a gas differs from the work of a gas only in sign  , because the strength
, because the strength  acting on the gas is opposite to the force
acting on the gas is opposite to the force  , with which the gas acts on the piston, and is equal to it in absolute value (Newton's third law); and the movement remains the same. Therefore, the work of external forces is equal to:
, with which the gas acts on the piston, and is equal to it in absolute value (Newton's third law); and the movement remains the same. Therefore, the work of external forces is equal to:
 .
.
First law of thermodynamics:
The first law of thermodynamics is the law of conservation of energy, extended to thermal phenomena. Law of energy conservation: energy in nature does not arise from nothing and does not disappear: the amount of energy is unchanged, it only changes from one form to another.
In thermodynamics, bodies are considered, the position of the center of gravity of which practically does not change. The mechanical energy of such bodies remains constant, and only the internal energy can change.
Internal energy can be changed in two ways: heat transfer and work. In the general case, the internal energy changes both due to heat transfer and due to the performance of work. The first law of thermodynamics is formulated precisely for such general cases:
The change in the internal energy of the system during its transition from one state to another is equal to the sum of the work of external forces and the amount of heat transferred to the system:

If the system is isolated, then no work is done on it and it does not exchange heat with the surrounding bodies. According to the first law of thermodynamics the internal energy of an isolated system remains unchanged.
Given that  , the first law of thermodynamics can be written as follows:
, the first law of thermodynamics can be written as follows:

The amount of heat transferred to the system goes to change its internal energy and to perform work on external bodies by the system.
Second law of thermodynamics: it is impossible to transfer heat from a colder system to a hotter one in the absence of other simultaneous changes in both systems or in the surrounding bodies.