Objective
Assimilation and consolidation of theoretical material on the topic of the thermodynamics course "Water vapor", as well as mastering the methods of experiment and processing of the data obtained, familiarization with the tables "Thermophysical properties of water and steam".
1. Study the scheme of the experimental setup, turn it on and bring it to a given stationary thermal regime.
2. Carry out the experiment in accordance with the guidelines, fill in table 1.
3. Determine the specific heat spent on the vaporization of water in the experiment.
4. For the isobaric process of vaporization, determine the tabular values of the parameters of water on the saturation line and dry saturated steam, as well as the specific heat of vaporization.
5. Calculate the internal energy of the liquid on the vapor saturation line for the conditions of the experiment.
6. Calculate the error of the found value specific heat vaporization in relation to the table.
7. Depict the processes occurring in the Dewar vessel in P-v and T-s-diagrams.
8. Make a conclusion on the work.
METHODOLOGICAL INSTRUCTIONS
The transition of a substance from a liquid state to a gaseous state is called vaporization, the reverse transition is called condensation. Boiling a liquid is a process of vaporization inside a liquid that occurs at a strictly defined temperature t n, ° C, determined by pressure. If a gaseous phase exists with a liquid phase of the same substance, then it is called vapor. The gaseous phase of the system is dry saturated steam, and the liquid phase is a liquid that retains the state corresponding to the beginning of vaporization.
During vaporization according to the isobaric-isothermal process, according to the first law of thermodynamics, the specific heat of phase transformation (specific heat of vaporization) r, J / kg,
r \u003d u "- u" + p (v "-v"), (1)
r = i" - i" , (2)
where u", i", v" - respectively, internal energy, enthalpy, J / kg, and the specific volume of dry saturated steam, m 3 / kg;
u", i", v" - respectively, the internal energy, enthalpy, J / kg, and the specific volume of the liquid in the state of saturation, m 3 / kg.
The pressure p, Pa, is not marked with special indices, since it does not change during the entire phase transition and is equal to the saturation pressure.
Thus, the specific heat of vaporization includes a change in the internal energy of a substance and the work of a change in volume during a phase transition.
The specific heat of vaporization is functionally related to the state parameters. For most substances used in practice, the properties of liquid and vapor on the saturation line are determined and tabulated. These tables give the values of p and t on the saturation line and the corresponding values of v", v", i", i", r, s", s". The internal energy of the liquid on the saturation line u", J / kg, and dry saturated steam u", J / kg, is determined respectively by the equations
u"=i"-pv"(3)
u" = i" -pv" (4)
EXPERIMENTAL SETUP
Drawing. Scheme of the experimental setup
The experimental setup (figure) consists of a Dewar vessel 1 with an electric heater 2, into which a portion of distilled water is poured from a container 3, regulated by a valve 4. The resulting vapor in the condenser 5, through which tap water passes, turns into a liquid. The water flow is regulated by the valve 7 according to the control lamp 8. The resulting condensate is collected in a measuring cylinder 9. On the control panel are: switch "NETWORK" 10, voltmeter 11, ammeter 12, mode switch 13; 6 - glass funnel.
EXPERIMENTAL TECHNIQUE
1. Turn the unit on by turning switch 10 to position "1".
2. Check the filling of the Dewar vessel 1 by setting the mode switch 13 to the "FILLING" position. If at the same time the green signal lamp "Vessel is full" lights up, you can start the experiment. Otherwise, the vessel is filled with distilled water, for which valve 4 is opened. After the green signal lamp lights up, close the vessel tightly.
3. Move switch 13 to the "HEATING" position.
4. Turning the knob of the autotransformer 14, set the value of the voltage on the heater U, V (and the current strength I, A) set by the teacher.
5. Supply cooling water to the condenser 5 by opening the valve 7 and adjust the water flow according to the control lamp 8.
6. When a stationary mode of water boiling in a Dewar vessel is established (15-20 cm of condensate will accumulate in measuring cylinder 9), make a control collection of condensate in the amount indicated by the teacher (V, m 3). The duration of the control collection t, s, is determined by the stopwatch.
7. Using a barometer, determine the atmospheric pressure P a, mm Hg.
8. Enter the measurement data into the table of observations and sign it with the teacher.
9. Turn on the unit by turning the switch "0", close valve 7, turn the handle of the autotransformer counterclockwise until it stops, drain the condensate into container 3.
Table 1
|
Measurement number |
mm. rt. Art. | ||||
PROCESSING OF EXPERIMENTAL DATA
1. Calculate the amount of heat spent on vaporization of 1 kg of water r op, J / kg:
r op = (W - Q) / (Vr),
where W = UI - heater power, W;
Q = 0.04W - heat losses, W;
r is the density of the condensate, kg / m 3. We accept r \u003d 1000 kg / m 3.
2. Assuming that water boils at atmospheric pressure, determine from the tabular values of the water parameters on the saturation line and dry saturated steam, which are entered in Table 2.
Table 2
|
i", kJ/kg |
S", kJ/(kgK) |
i", kJ/kg |
S", J/(kgK) | |||
3. Calculate the values of the internal energy of water on the saturation line u" and dry saturated steam u", kJ/kg, using formulas (3) and (4).
4. Calculate the error, %, of the found value of the specific heat of vaporization r op, kJ / kg, in relation to the tabular r, kJ / kg, according to the formula:
D \u003d (r op - r) 100 / r.
5. Present graphically the processes occurring in the Dewar vessel in P-v and T-s-diagrams.
6. Make a conclusion on the work.
QUESTIONS FOR SELF-EDUCATION
1. Vaporization of liquid; the essence of the processes of boiling and evaporation of a liquid.
2. Isobaric process of transition of liquid into superheated steam in P-v and T-s-diagrams.
3. Boundary curves with the degree of dryness x = 0 and x = 1, the critical state of the substance
4. Concepts: liquid on the saturation line, wet saturated steam, dry saturated steam, superheated steam.
5. Specific heat of vaporization of liquid.
6. The degree of dryness, the degree of humidity of the steam.
7. Tables of thermophysical properties of water and water vapor, their meaning.
8. Determination of wet steam parameters.
9. i-s-diagram of water vapor, its purpose.
10. Steam thermodynamic processes in P-v, T-s, i-s-diagrams.
REFERENCES
1. Heat engineering / Ed. A.P. Baskakova.- M.: Energoizdat, 1991.- 224 p.
2. Nashchokin V.V. Technical thermodynamics and heat transfer.- M.:: graduate School, 1980.- 496 p.
3. Yudaev B.N. Technical thermodynamics. Heat transfer. - M .: Higher school, 1998. - 480 p.
4. Rivkin S.L., Aleksandrov A.A. Tables of thermophysical properties of water and steam.- M.: Energy, 1980.- 408 p.
Do you know what the temperature of the boiled soup is? 100 ˚С. No more, no less. At the same temperature, the kettle boils and the pasta is boiled. What does it mean?
Why does the temperature of the water inside not rise above one hundred degrees when a saucepan or kettle is constantly heated with burning gas? The fact is that when water reaches a temperature of one hundred degrees, all the incoming thermal energy is spent on the transition of water into a gaseous state, that is, evaporation. Up to a hundred degrees, evaporation occurs mainly from the surface, and when it reaches this temperature, the water boils. Boiling is also evaporation, but only over the entire volume of the liquid. Hot steam bubbles are formed inside the water and, being lighter than water, these bubbles break out to the surface, and the steam from them escapes into the air.
Up to a hundred degrees, the temperature of the water rises when heated. After a hundred degrees, with further heating, the temperature of the water vapor will increase. But until all the water boils away at one hundred degrees, its temperature will not rise, no matter how much energy you apply. We have already figured out where this energy goes - to the transition of water into a gaseous state. But if such a phenomenon exists, then there must be the physical quantity that describes this phenomenon. And such a value exists. It is called the specific heat of vaporization.
Specific heat of vaporization of water
The specific heat of vaporization is a physical quantity that indicates the amount of heat required to turn a 1 kg liquid into vapor at the boiling point. The specific heat of vaporization is denoted by the letter L. And the unit of measurement is the joule per kilogram (1 J / kg).
The specific heat of vaporization can be found from the formula:
where Q is the amount of heat,
m - body weight.
By the way, the formula is the same as for calculating the specific heat of fusion, the difference is only in the designation. λ and L
Empirically, the values of the specific heat of vaporization of various substances were found and tables were compiled from which data can be found for each substance. Thus, the specific heat of vaporization of water is 2.3*106 J/kg. This means that for every kilogram of water, an amount of energy equal to 2.3 * 106 J must be spent to turn it into steam. But at the same time, the water should already have a boiling point. If the water was initially at a lower temperature, then it is necessary to calculate the amount of heat that will be required to heat the water to one hundred degrees.
In real conditions, it is often necessary to determine the amount of heat required for the transformation of a certain mass of a liquid into vapor, therefore, more often one has to deal with a formula of the form: Q \u003d Lm, and the values \u200b\u200bof the specific heat of vaporization for a particular substance are taken from ready-made tables.
In this lesson, we will pay attention to such a type of vaporization as boiling, discuss its differences from the previously considered evaporation process, introduce such a value as the boiling point, and discuss what it depends on. At the end of the lesson, we will introduce a very important quantity that describes the process of vaporization - the specific heat of vaporization and condensation.
Topic: Aggregate states of matter
Lesson: Boil. Specific heat of vaporization and condensation
In the last lesson, we have already considered one of the types of vaporization - evaporation - and highlighted the properties of this process. Today we will discuss such a type of vaporization as the boiling process, and introduce a value that numerically characterizes the vaporization process - the specific heat of vaporization and condensation.
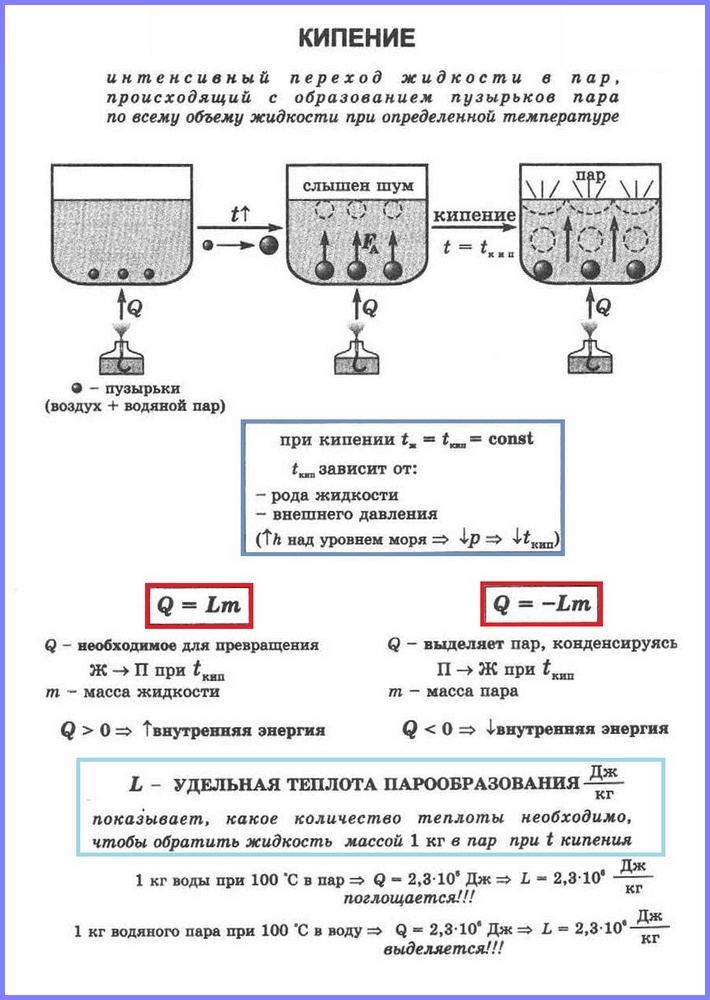
Definition.Boiling(Fig. 1) is a process of intensive transition of a liquid into a gaseous state, accompanied by the formation of vapor bubbles and occurring throughout the volume of the liquid at a certain temperature, which is called the boiling point.
Let's compare two types of vaporization with each other. The boiling process is more intense than the evaporation process. In addition, as we remember, the evaporation process takes place at any temperature above the melting point, and the boiling process - strictly at a certain temperature, which is different for each of the substances and is called the boiling point. It should also be noted that evaporation occurs only from the free surface of the liquid, i.e., from the area that delimits it from the surrounding gases, and boiling occurs immediately from the entire volume.
Let us consider the course of the boiling process in more detail. Let's imagine a situation that many of us have repeatedly encountered - this is heating and boiling water in a certain vessel, for example, in a saucepan. During heating, a certain amount of heat will be transferred to the water, which will lead to an increase in its internal energy and an increase in the activity of molecular movement. This process will proceed up to a certain stage, until the energy of molecular motion becomes sufficient to start boiling.
Dissolved gases (or other impurities) are present in water, which are released in its structure, which leads to the so-called emergence of centers of vaporization. That is, it is in these centers that steam is released, and bubbles form throughout the entire volume of water, which are observed during boiling. It is important to understand that these bubbles are not air, but steam, which is formed during the boiling process. After the formation of bubbles, the amount of vapor in them increases, and they begin to increase in size. Often, bubbles initially form near the walls of the vessel and do not immediately rise to the surface; first, they, increasing in size, are under the influence of the growing force of Archimedes, and then break away from the wall and rise to the surface, where they burst and release a portion of steam.
It should be noted that not all steam bubbles reach the free surface of the water at once. At the beginning of the boiling process, the water is still far from evenly heated, and the lower layers, near which the heat transfer process takes place, are even hotter than the upper ones, even taking into account the convection process. This leads to the fact that the steam bubbles rising from below collapse due to the phenomenon of surface tension, not yet reaching the free surface of the water. At the same time, the steam that was inside the bubbles passes into the water, thereby additionally heating it and accelerating the process of uniform heating of the water throughout the volume. As a result, when the water is heated almost evenly, almost all steam bubbles begin to reach the surface of the water and the process of intense vaporization begins.
It is important to highlight the fact that the temperature at which the boiling process takes place remains unchanged even if the intensity of heat supply to the liquid is increased. In simple words If, during the boiling process, gas is added to the burner, which heats the pot of water, this will only increase the intensity of the boil, and not increase the temperature of the liquid. If we delve more seriously into the boiling process, it is worth noting that there are areas in water in which it can be overheated above the boiling point, but the magnitude of such overheating, as a rule, does not exceed one or a couple of degrees and is insignificant in the total volume of the liquid. The boiling point of water at normal pressure is 100°C.
In the process of boiling water, you can notice that it is accompanied by characteristic sounds of the so-called seething. These sounds arise just because of the described process of collapse of steam bubbles.
The processes of boiling other liquids proceed in the same way as the boiling of water. The main difference in these processes is the different boiling points of substances, which at normal atmospheric pressure are already measured tabular values. Let us indicate the main values of these temperatures in the table.
An interesting fact is that the boiling point of liquids depends on the value of atmospheric pressure, which is why we indicated that all values in the table are given at normal atmospheric pressure. When the air pressure increases, the boiling point of the liquid also increases, and when it decreases, on the contrary, it decreases.
On this dependence of boiling point on pressure environment the principle of operation of such a well-known kitchen appliance as a pressure cooker is based (Fig. 2). It is a pan with a tight-fitting lid, under which, in the process of water vaporization, the air pressure with steam reaches values up to 2 atmospheric pressures, which leads to an increase in the boiling point of water in it up to . Because of this, the water with the food in it has the opportunity to heat up to a temperature higher than usual (), and the cooking process is accelerated. Because of this effect, the device got its name.

Rice. 2. Pressure cooker ()
The situation with a decrease in the boiling point of a liquid with a decrease in atmospheric pressure also has an example from life, but no longer everyday for many people. This example applies to the travel of climbers in the highlands. It turns out that in an area located at an altitude of 3000-5000 m, the boiling point of water, due to a decrease in atmospheric pressure, decreases to even lower values, which leads to difficulties in cooking on hikes, since in this case, effective thermal processing of products requires much more time than under normal conditions. At altitudes of about 7000 m, the boiling point of water reaches , which makes it impossible to cook many products in such conditions.
Some technologies for the separation of substances are based on the fact that the boiling points of various substances are different. For example, if we consider the heating of oil, which is a complex liquid consisting of many components, then in the process of boiling it can be divided into several different substances. IN this case, due to the fact that the boiling points of kerosene, gasoline, naphtha and fuel oil are different, they can be separated from each other by vaporization and condensation at different temperatures. This process is usually referred to as fractionation (Fig. 3).

Rice. 3 Separation of oil into fractions ()
Like any physical process, boiling must be characterized using some numerical value, such a value is called the specific heat of vaporization.
In order to understand the physical meaning of this value, consider the following example: take 1 kg of water and bring it to the boiling point, then measure how much heat is needed to completely evaporate this water (excluding heat losses) - this value will be equal to the specific heat of vaporization of water. For another substance, this value of heat will be different and will be the specific heat of vaporization of this substance.
The specific heat of vaporization turns out to be a very important characteristic in modern technologies metal production. It turns out that, for example, during the melting and evaporation of iron, followed by its condensation and solidification, crystal cell with a structure that provides higher strength than the original sample.
Designation: specific heat of vaporization and condensation (sometimes denoted by ).
Unit: .
The specific heat of vaporization of substances is determined by experiments in laboratory conditions, and its values for the main substances are listed in the appropriate table.
|
Substance |
The process of changing a substance from a liquid state to a gaseous state is called vaporization. Vaporization can be carried out in the form of two processes: i.
Boiling
The second process of vaporization is boiling. You can watch this process with simple experience heating water in a glass flask. When water is heated, bubbles appear in it after a while, which contain air and saturated water vapor, which is formed during the evaporation of water inside the bubbles. When the temperature rises, the pressure inside the bubbles increases, and under the action of the buoyancy force, they rise up. However, since the temperature of the upper layers of water is lower than the lower ones, the vapor in the bubbles begins to condense and they shrink. When the water warms up throughout the volume, the bubbles with steam rise to the surface, burst, and the steam comes out. Water is boiling. This occurs at a temperature at which the saturation vapor pressure in the bubbles is equal to atmospheric pressure.
The process of vaporization occurring in the entire volume of a liquid at a certain temperature is called. The temperature at which a liquid boils is called boiling point.
This temperature depends on atmospheric pressure. As atmospheric pressure rises, the boiling point rises.
Experience shows that during the boiling process the temperature of the liquid does not change, despite the fact that energy comes from outside. The transition of a liquid to a gaseous state at the boiling point is associated with an increase in the distance between the molecules and, accordingly, with overcoming the attraction between them. The energy supplied to the fluid is expended to do the work of overcoming the forces of attraction. This happens until all the liquid turns into vapor. Since liquid and vapor have the same temperature during the boiling process, the average kinetic energy of the molecules does not change, only their potential energy increases.
The figure shows a graph of water temperature versus time as it is heated from room temperature to boiling (AB), boiling (BC), steam heating (CD), steam cooling (DE), condensation (EF) and subsequent cooling (FG).
Specific heat of vaporization
For the transformation of different substances from a liquid state into a gaseous state, different energy is required, this energy is characterized by a value called the specific heat of vaporization.
Specific heat of vaporization (L) is a value equal to the ratio of the amount of heat that must be imparted to a substance with a mass of 1 kg to transform it from a liquid state into a gaseous state at the boiling point.
The unit of specific heat of vaporization is [ L] = J/kg.
To calculate the amount of heat Q, which must be imparted to a substance with a mass mn for its transformation from a liquid state to a gaseous one, it is necessary to have the specific heat of vaporization ( L) times the mass of the substance: Q = Lm.
When steam condenses, a certain amount of heat is released, and its value is equal to the value of the amount of heat that must be spent to turn the liquid into steam at the same temperature.
Specific heat
Specific heat capacity is the amount of heat in Joules (J) required to raise the temperature of a substance. Specific heat capacity is a function of temperature. For gases, a distinction must be made between specific heat at constant pressure and at constant volume.
Specific heat of fusion
The specific heat of fusion of a solid is the amount of heat in J required to convert 1 kg of a substance from a solid to a liquid state at the melting point.
Latent heat of vaporization
The latent heat of vaporization of a liquid is the amount of heat in J required to vaporize 1 kg of liquid at the boiling point. The latent heat of vaporization is highly dependent on pressure. Example: if heat is applied to a container containing 1 kg of water at 100°C (at sea level), the water will absorb 1023 kJ of latent heat without any change in the thermometer reading. However, there will be a change in the state of aggregation from liquid to vapor. The heat absorbed by water is called the latent heat of vaporization. Steam will save 1023 kJ, since this energy was required to change the state of aggregation.
Latent heat of condensation
In the reverse process, when heat is removed from 1 kg of water vapor at 100°C (at sea level), the steam will release 1023 kJ of heat without changing the thermometer readings. However, there will be a change in the state of aggregation from vapor to liquid. The heat absorbed by the water is called the latent heat of condensation.
Temperature and pressure

Thermal measurements
Temperature, or INTENSITY of heat, is measured with a thermometer. Most temperatures in this manual are given in degrees Celsius (C), but sometimes degrees Fahrenheit (F) are also used. The temperature value tells only about the intensity of heat or sensible HEAT, and not about the actual amount of heat. Comfortable temperature for a person is in the range from 21 to 27°C. In this temperature range, a person feels most comfortable. When any temperature is above or below this range, the person perceives it as warm or cold. In science, there is the concept of "absolute zero" - the temperature at which all heat is removed from the body. Absolute zero temperature is defined as -273°C. Any substance at a temperature above absolute zero contains some amount of heat. To understand the basics of air conditioning, it is also necessary to understand the relationship between pressure, temperature, and state of aggregation. Our planet is surrounded by air, in other words gas. The pressure in a gas is transmitted equally in all directions. The gas around us is 21% oxygen and 78% nitrogen. The remaining 1% is occupied by other rare gases. This combination of gases is called the atmosphere. It extends several hundred kilometers above the earth's surface and is held together by the force of gravity. At sea level, atmospheric pressure is 1.0 bar and the boiling point of water is 100°C. At any point above sea level, the atmospheric pressure is lower, and the boiling point of water is also lower. When the pressure is reduced to 0.38 bar, the boiling point of water is 75°C, and at a pressure of 0.12 bar - 50°C. If the boiling point of water is affected by a decrease in pressure, it is logical to assume that an increase in pressure will also affect it. An example is a steam boiler!
Additional information: how to convert degrees Fahrenheit to degrees Celsius and vice versa: C = 5/9 × (F - 32). F = (9/5 × C)+32. Kelvin = C + 273. Rankine = F + 460.